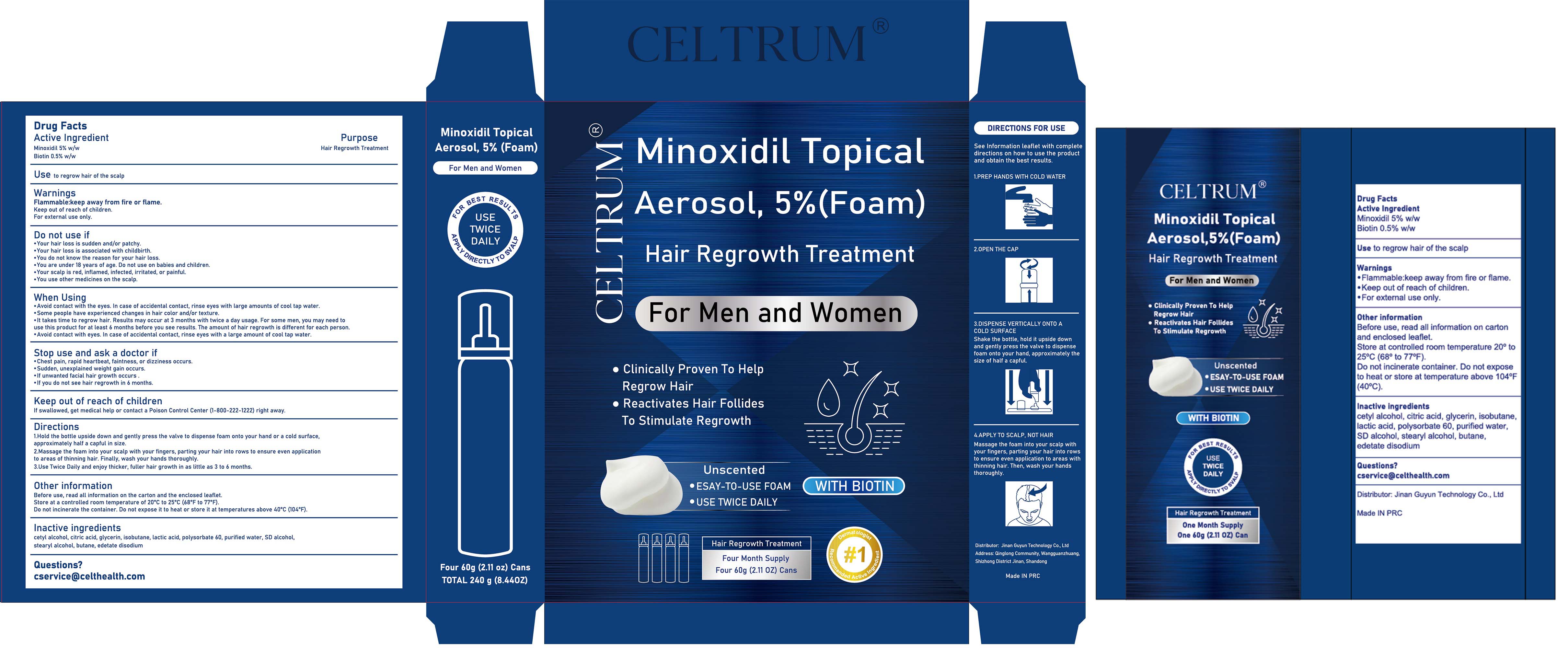
Label: 5% MINOXIDIL HAIR GROWTH FOAM WITH BIOTIN- minoxidil aerosol, foam
- NDC Code(s): 83988-003-01
- Packager: Jinan Guyun Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
-
WHEN USING
Avoid contact with the eyes. in case of accidental contact, rinse eyes with large amounts of cool tap water.
Some people have experienced changes in hair color and/or texture.
lt takes time to regrow hair. Results may occur at 3 months with twice a day usage. For some men, you may need touse this product for at least 6 months before you see results. The amount of hair regrowth is different for each person.
Avoid contact with eyes, in case of accidental contact, rinse eyes with a large amount of cool tap water. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
1.Hold the bottle upside down and gently press the valve to dispense foam onto your hand or a cold surface,approximately half a capful in size.
2.Massage the foam into your scalp with your fingers, parting your hair into rows to ensure even applicationto areas of thinning hair. Finally, wash your hands thoroughly.3.Use Twice Daily and enjoy thicker, fuller hair growth in as little as 3 to 6 months.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
5% MINOXIDIL HAIR GROWTH FOAM WITH BIOTIN
minoxidil aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83988-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 0.5 g in 100 g MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) CITRIC ACID (UNII: 2968PHW8QP) LACTIC ACID (UNII: 33X04XA5AT) POLYSORBATE 60 (UNII: CAL22UVI4M) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CETYL ALCOHOL (UNII: 936JST6JCN) BUTANE (UNII: 6LV4FOR43R) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) ISOBUTANE (UNII: BXR49TP611) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83988-003-01 4 in 1 PACKAGE 12/12/2024 1 60 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/12/2024 Labeler - Jinan Guyun Technology Co., Ltd (544454964) Establishment Name Address ID/FEI Business Operations Jinan Guyun Technology Co., Ltd 544454964 manufacture(83988-003)