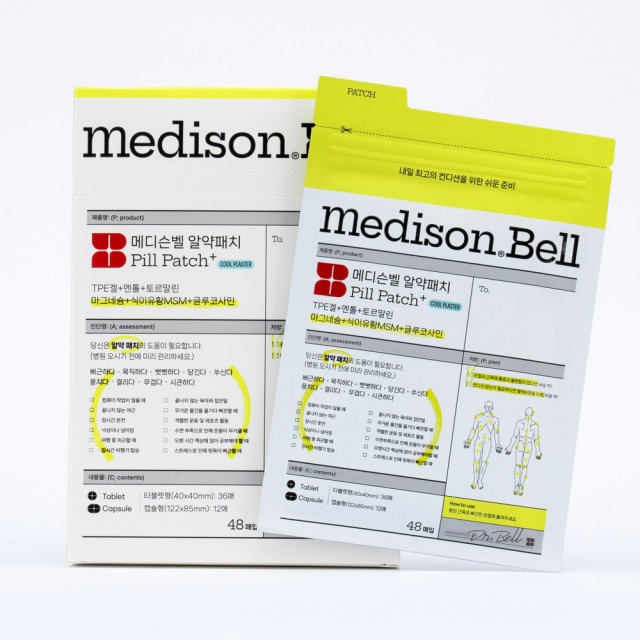
Label: MEDISON BELL PILL PATCH- tourmaline, magnesium sulfate, dimethyl sulfone, glucosamine kit
- NDC Code(s): 71829-0010-1
- Packager: SENNY STUDIO Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
1. Do not use if you have skin conditions, eczema, or allergies.
2. If you experience swelling, pain, or red spots on the skin during use, stop using immediately and consult a physician.
3. Recommended use is up to 8 hours per day.
4. Store any unused patches in the resealable pouch to maintain effectiveness.
5. Keep out of reach of infants and children.
6. Do not use for purposes other than intended, and keep away from fire.
7. Avoid storing in temperatures above 30°C or in direct sunlight. - DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEDISON BELL PILL PATCH
tourmaline, magnesium sulfate, dimethyl sulfone, glucosamine kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71829-0010 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71829-0010-1 1 in 1 CARTON 12/11/2024 1 1 in 1 POUCH; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 36 PATCH 36 Part 2 12 PATCH 12 Part 1 of 2 MEDISON BELL PILL PATCHTABLET
tourmaline, magnesium sulfate, dimethyl sulfone, glucosamine liquidProduct Information Item Code (Source) NDC:71829-0011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLUCOSAMINE (UNII: N08U5BOQ1K) (GLUCOSAMINE - UNII:N08U5BOQ1K) GLUCOSAMINE 0.0045 g DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) (DIMETHYL SULFONE - UNII:9H4PO4Z4FT) DIMETHYL SULFONE 0.0045 g MAGNESIUM SULFATE (UNII: DE08037SAB) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE 0.0135 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) MENTHOL (UNII: L7T10EIP3A) Product Characteristics Color Score Shape ROUND (40x40mm) Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other Part 2 of 2 MEDISON BELL PILL PATCHCAPSULE
tourmaline, magnesium sulfate, dimethyl sulfone, glucosamine liquidProduct Information Item Code (Source) NDC:71829-0012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLUCOSAMINE (UNII: N08U5BOQ1K) (GLUCOSAMINE - UNII:N08U5BOQ1K) GLUCOSAMINE 0.0155 g DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) (DIMETHYL SULFONE - UNII:9H4PO4Z4FT) DIMETHYL SULFONE 0.0155 g MAGNESIUM SULFATE (UNII: DE08037SAB) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE 0.045 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) MENTHOL (UNII: L7T10EIP3A) Product Characteristics Color Score Shape CAPSULE (122x85mm) Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/11/2024 Labeler - SENNY STUDIO Co., Ltd (557827314) Registrant - SENNY STUDIO Co., Ltd (557827314) Establishment Name Address ID/FEI Business Operations SENNY STUDIO Co., Ltd 557827314 manufacture(71829-0010)