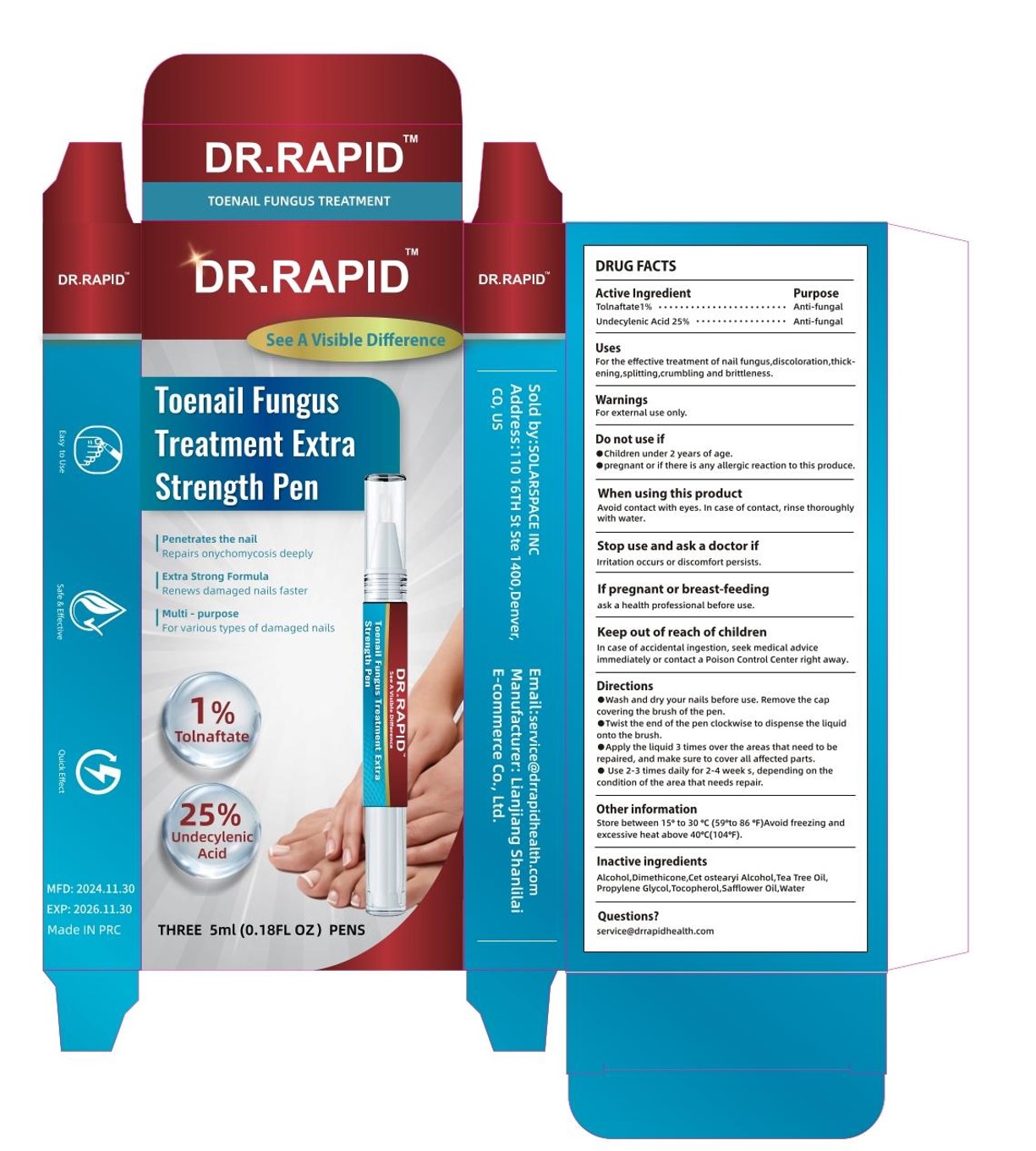
Label: TOENAIL FUNGUS TREATMENT EXTRA STRENGTH PEN- tolnaftate, undecylenic acid liquid
- NDC Code(s): 85038-001-01
- Packager: Lianjiang Shanlilai E-commerce Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Wash and dry your nails before use, Remove the capcovering the brush of the pen.
Twist the end of the pen clockwise to dispense the liquidonto the brush.
Apply the liquid 3 times over the areas that need to berepalred, and make sure to cover all affected parts.
Use 2-3 times daily for 2-4 week s, depending on thecondition of the area that needs repair. - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TOENAIL FUNGUS TREATMENT EXTRA STRENGTH PEN
tolnaftate, undecylenic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:85038-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 mL UNDECYLENIC ACID (UNII: K3D86KJ24N) (UNDECYLENIC ACID - UNII:K3D86KJ24N) UNDECYLENIC ACID 25 g in 100 mL Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) SAFFLOWER OIL (UNII: 65UEH262IS) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALCOHOL (UNII: 3K9958V90M) TEA TREE OIL (UNII: VIF565UC2G) DIMETHICONE (UNII: 92RU3N3Y1O) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:85038-001-01 3 in 1 PACKAGE 12/11/2024 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 12/11/2024 Labeler - Lianjiang Shanlilai E-commerce Co., Ltd. (638192827) Establishment Name Address ID/FEI Business Operations Lianjiang Shanlilai E-commerce Co., Ltd. 638192827 manufacture(85038-001)