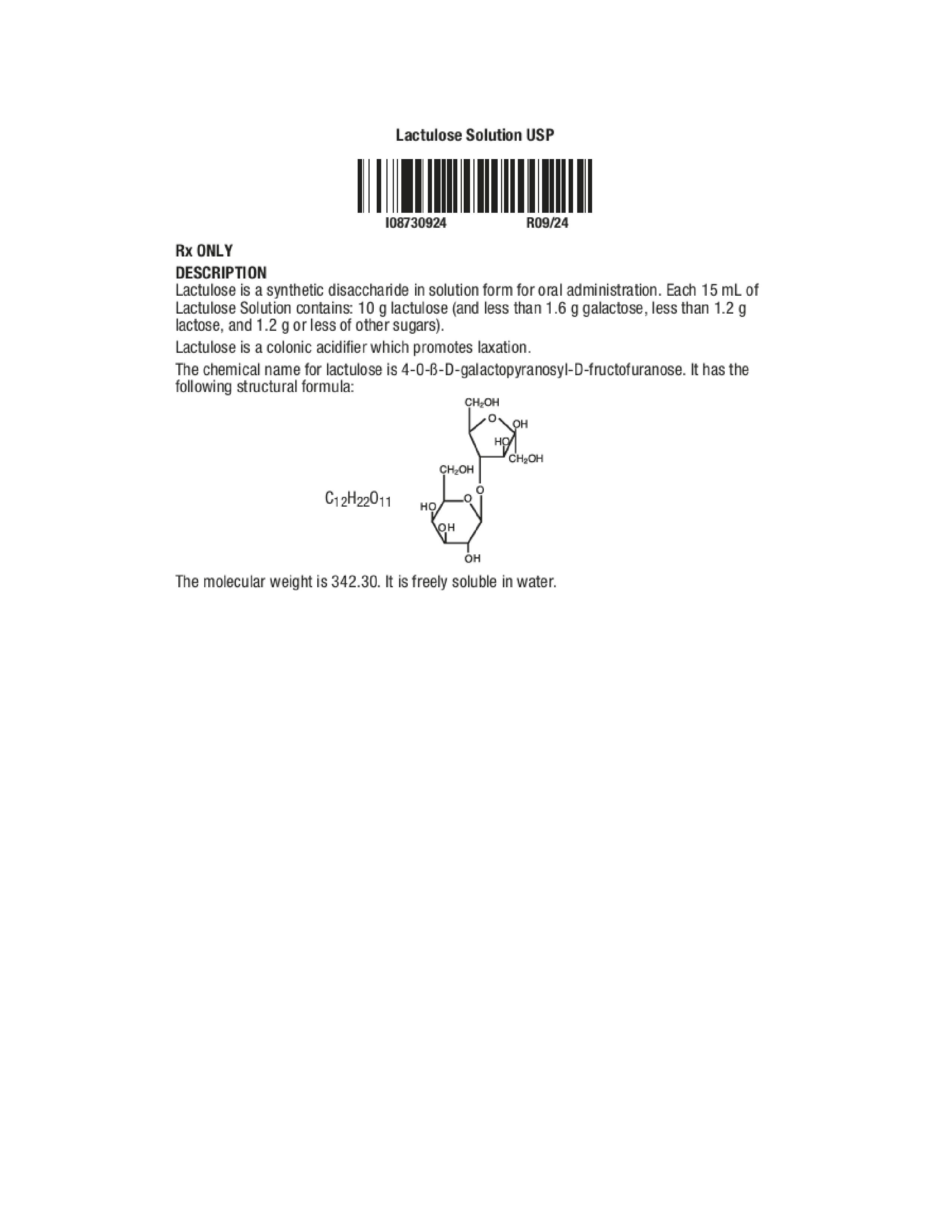
Label: LACTULOSE liquid
- NDC Code(s): 80432-068-33, 80432-068-35
- Packager: TriRx Huntsville Pharmaceutical Services
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Description
- Clinical Pharmacology
- Indications and Usage
- Contraindications
- Warnings
- Precautions
- Pregnancy
- Pediatric Use
- Adverse Reactions
- Overdosage
- Dosage Administration
- How Supplied
- Label 16 oz
- Label 32 oz
-
INGREDIENTS AND APPEARANCE
LACTULOSE
lactulose liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:80432-068 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LACTULOSE (UNII: 9U7D5QH5AE) (LACTULOSE - UNII:9U7D5QH5AE) LACTULOSE 10 g in 15 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80432-068-33 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/10/2024 2 NDC:80432-068-35 946 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074623 12/10/2024 Labeler - TriRx Huntsville Pharmaceutical Services (117090286)