Label: DIAL PROFESSIONAL CLEAN AND GENTLE ANTIBACTERIAL FOAMING HAND WASH- dial professional clean and gentle antibacterial foaming hand soap liquid
DIAL PROFESSIONAL ORIGINAL ANTIBACTERIAL DEFENSE FOAMING HAND WASH PLUS ALOE liquid
DIAL PROFESSIONAL SENSITIVE SKIN ANTIBACTERIAL- dial professional sensitive skin antibacterial liquid hand soap liquid
DIAL PROFESSIONAL ANTIBACTERIAL HAND WASH SPRING WATER SCENT- dial professional antibacterial hand soap spring water scent liquid
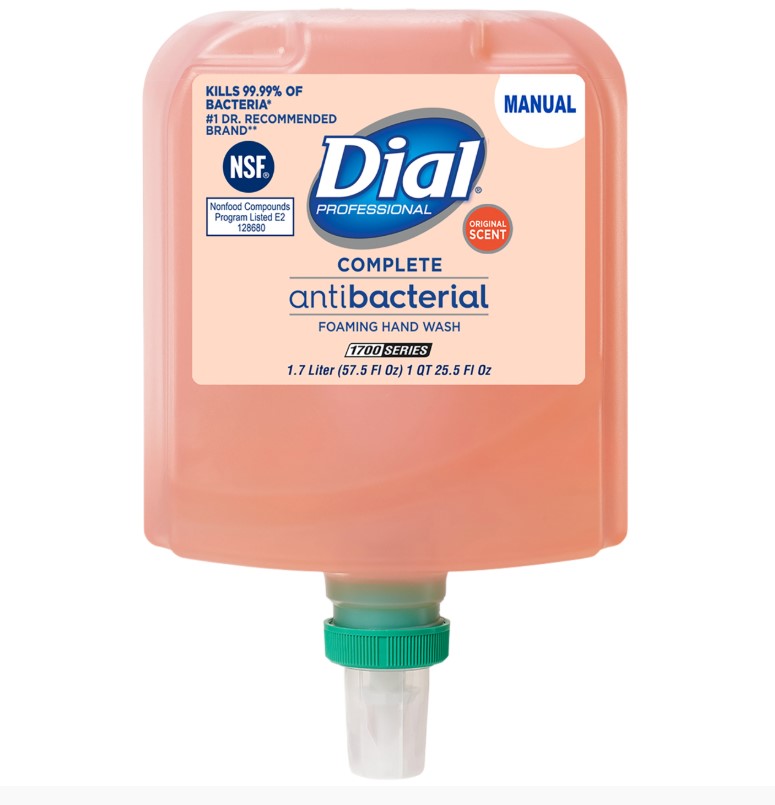
DIAL PROFESSIONAL COMPLETE ANTIBACTERIAL FOAMING HAND WASH ORIGINAL SCENT liquid
DIAL PROFESSIONAL BASICS HYPOALLERGENIC FOAMING HAND WASH1 GALLON REFILL liquid
DIAL PROFESSIONAL COMPLETE ORIGINAL ANTIMICROBIAL FOAMING WASH- dial professional complete original antimicrobial foaming hand wash liquid
DIAL PROFESSIONAL DIAL COMPLETE SPRING WATER ANTIBACTERIAL FOAMING HAND WASH REFILL liquid
DIAL PROFESSIONAL ANTIBACTERIAL FOAMING HAND WASH SPRING WATER- dial professional antibacterial foaming hand soap spring water scent liquid
-
NDC Code(s):
54340-137-01,
54340-137-02,
54340-138-01,
54340-138-02, view more54340-138-03, 54340-138-04, 54340-138-05, 54340-138-06, 54340-138-07, 54340-138-08, 54340-139-01, 54340-139-02, 54340-140-01, 54340-140-02, 54340-140-03, 54340-140-04, 54340-140-05, 54340-140-06, 54340-196-01, 54340-196-02, 54340-196-03, 54340-196-04, 54340-196-05, 54340-196-06, 54340-196-07, 54340-196-08, 54340-196-09, 54340-196-10, 54340-196-11, 54340-196-12, 54340-263-01, 54340-263-02, 54340-287-01, 54340-287-02, 54340-288-01, 54340-288-02, 54340-288-03, 54340-288-04, 54340-288-05, 54340-288-06, 54340-289-01, 54340-289-02
- Packager: Henkel Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Dial® Professional Complete Original Antibacterial Dial 1700 Universal Manual Refill
Dial® Professional Sensitive Skin Antibacterial Liquid Hand Soap
Dial Prof Antibacterial Liquid Hand Soap Spring Water Refill
Dial® Professional Complete Original Antibacterial Dial 1700 Universal Manual Refill
Aqua (Water, Eau)
Glycerin
Lauramine Oxide
Cetrimonium Chloride
Cocamidopropyl Betaine
DMDM HydantoinPEG-9
Zinc Sulfate
Aloe Barbadensis Leaf Juice
Citric Acid
Sodium Chloride
Dimethyl Lauramine
Tetrasodium EDTA
Parfum (Fragrance)
Alcohol
Hydroxypropyl Methylcellulose
Dimethyl Myristamine
Sunflowerseedamidopropyl Ethyldimonium Ethosulfate
CI 14700 (Red 4)
CI 19140 (Yellow 5)Dial® Professional Sensitive Skin Antibacterial Liquid Hand Soap
Aqua (Water, Eau)
Lauramidopropylamine Oxide
Glycerin
Lauramine Oxide
Cetrimonium Chloride
Sodium Chloride
PEG-120 Methyl Glucose Dioleate
Citric Acid
Sodium Benzoate
Zinc Sulfate
Myristamidopropylamine Oxide
Parfum (Fragrance)
Dimethyl Lauramine
Tetrasodium EDTA
Hydrogen Peroxide
Alcohol
Dimethyl MyristamineDial Professional Antibacterial Liquid Hand Soap Spring Water Refill
Aqua (Water, Eau)
Lauramidopropylamine Oxide
Glycerin
Lauramine Oxide
Cetrimonium Chloride
Sodium Chloride
PEG-120 Methyl Glucose Dioleate
Citric Acid
Sodium Benzoate
Zinc Sulfate
Myristamidopropylamine Oxide
Parfum (Fragrance)
Dimethyl Lauramine
Tetrasodium EDTA
Alcohol
Dimethyl Myristamine
CI 42090 (Blue 1)
CI 17200 (Red 33) -
Warnings
Warnings For external use only
When using this product•Avoid contact with eyes •
Stop use and ask a doctor if•Irritation or redness develops OR Stop use and ask a doctor ifirritation or redness develops
Keep out of reach of children. •If swallowed, get medical help or contact a Poison Control Center right away
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- Dial Professional Original Gold Antibacterial Liquid Hand Soap
- KEEP OUT OF REACH OF CHILDREN
- Purpose
- Dial® Professional Dial Complete Original Antibacterial Dial 1700 Universal Manual Refill - 3/1.7L Dial® Professional Dial Complete Original Antimicrobial FHW Manual Total Clean Dial® Professional Sensitive Skin Antibacterial Liquid Hand Soap - 12/7.5oz Decor Pump Dial® Professional Sensitive Skin Antibacterial Liquid Hand Soap - 12/16oz Pump Dial® Professional Sensitive Skin Antibacterial Liquid Hand Soap - Refill 8/1L Dial® Professional Sensitive Skin Antibacterial Liquid Hand Soap - Refill 4/1Gallon Dial® Professional Spring Water Antibacterial Liquid Hand Soap - Refill 4/1GallonDial Complete Spring Water Dial® Professional Antibacterial Dial 1700 Manual Refill - 3/1.7L Dial® Professional Dial Complete Spring Water FIT X1 Manual - Refill 3/1.2L Dial® Professional Dial Complete Spring Water FIT X2 Manual - Refill 3/1.2L Dial® Professional Dial Complete Spring Water FIT X2 Touch Free - Refill 3/1L Dial® Professional Clean + Gentle FIT Universal Manual Refill 3/1.2 L Dial® Professional Clean + Gentle FIT Universal Touch-Free Refill 3/1 L Dial® Professional Clean + Gentle Dial 1700 Refill 3/1.7 L Dial® Professional Clean + Gentle FIT X1 Manual Refill 3/1.2 L Dial® Professional Clean + Gentle FIT X1 Touch-Free Refill 3/1 L Dial® Professional Clean + Gentle FIT X2 Manual Refill 3/1.2 L Dial® Professional Clean + Gentle FIT X2 Touch-Free Refill 3/1 L Dial® Professional Complete Antibacterial Foaming Hand Wash Original Scent Manual Dial® Professional Complete Antibacterial Foaming Hand Wash Original Scent Dial® Professional Antimicrobial Liquid Hand Soap Dial® Professional Complete Antibacterial Foaming Hand Wash Original Scent Dial® Professional Complete Antibacterial Foaming Hand Wash Original Scent Manual
-
INGREDIENTS AND APPEARANCE
DIAL PROFESSIONAL CLEAN AND GENTLE ANTIBACTERIAL FOAMING HAND WASH
dial professional clean and gentle antibacterial foaming hand soap liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-288 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 95 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-288-02 3 in 1 CARTON 12/01/2020 1 NDC:54340-288-01 1200 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 2 NDC:54340-288-04 3 in 1 CARTON 12/01/2020 2 NDC:54340-288-03 1000 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 3 NDC:54340-288-06 3 in 1 CARTON 12/01/2020 3 NDC:54340-288-05 1700 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/01/2020 DIAL PROFESSIONAL ORIGINAL ANTIBACTERIAL DEFENSE FOAMING HAND WASH PLUS ALOE
dial professional original antibacterial defense foaming hand wash plus aloe liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-263 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 95.7 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-263-02 4 in 1 CARTON 01/01/2024 1 NDC:54340-263-01 3785 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/01/2024 DIAL PROFESSIONAL SENSITIVE SKIN ANTIBACTERIAL
dial professional sensitive skin antibacterial liquid hand soap liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-138 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 89.5 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-138-02 12 in 1 CARTON 01/01/2017 1 NDC:54340-138-01 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:54340-138-04 12 in 1 CARTON 01/01/2017 2 NDC:54340-138-03 473 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 3 NDC:54340-138-06 8 in 1 CARTON 01/01/2017 3 NDC:54340-138-05 1000 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 4 NDC:54340-138-08 4 in 1 CARTON 01/01/2017 4 NDC:54340-138-07 3785 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/01/2017 DIAL PROFESSIONAL ANTIBACTERIAL HAND WASH SPRING WATER SCENT
dial professional antibacterial hand soap spring water scent liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-139 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.3 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-139-02 4 in 1 CARTON 01/01/2017 1 NDC:54340-139-01 3785 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/01/2017 DIAL PROFESSIONAL COMPLETE ANTIBACTERIAL FOAMING HAND WASH ORIGINAL SCENT
dial professional complete antibacterial foaming hand wash original scent liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-196 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 95 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-196-02 3 in 1 CARTON 12/01/2022 1 NDC:54340-196-01 1200 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 2 NDC:54340-196-04 3 in 1 CARTON 12/01/2022 2 NDC:54340-196-03 1000 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 3 NDC:54340-196-06 8 in 1 CARTON 12/01/2022 3 NDC:54340-196-05 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 4 NDC:54340-196-08 12 in 1 CARTON 12/01/2022 4 NDC:54340-196-07 221 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 5 NDC:54340-196-10 4 in 1 CARTON 12/01/2022 5 NDC:54340-196-09 449 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 6 NDC:54340-196-12 3 in 1 CARTON 12/01/2022 6 NDC:54340-196-11 1200 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/01/2022 DIAL PROFESSIONAL BASICS HYPOALLERGENIC FOAMING HAND WASH1 GALLON REFILL
dial professional basics hypoallergenic foaming hand wash1 gallon refill liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-289 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 95.9 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-289-02 4 in 1 CARTON 01/01/2024 1 NDC:54340-289-01 3785 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/01/2024 DIAL PROFESSIONAL COMPLETE ORIGINAL ANTIMICROBIAL FOAMING WASH
dial professional complete original antimicrobial foaming hand wash liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-137 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 93.86 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-137-02 3 in 1 CARTON 11/01/2018 1 NDC:54340-137-01 1700 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 11/01/2018 DIAL PROFESSIONAL DIAL COMPLETE SPRING WATER ANTIBACTERIAL FOAMING HAND WASH REFILL
dial professional dial complete spring water antibacterial foaming hand wash refill liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-287 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 95.6 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-287-02 4 in 1 CARTON 01/01/2024 1 NDC:54340-287-01 3785 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/01/2024 DIAL PROFESSIONAL ANTIBACTERIAL FOAMING HAND WASH SPRING WATER
dial professional antibacterial foaming hand soap spring water scent liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-140 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.3 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-140-02 3 in 1 CARTON 09/01/2021 1 NDC:54340-140-01 1700 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 2 NDC:54340-140-04 3 in 1 CARTON 09/01/2021 2 NDC:54340-140-03 1200 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 3 NDC:54340-140-06 3 in 1 CARTON 09/01/2021 3 NDC:54340-140-05 1000 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/01/2021 Labeler - Henkel Corporation (080887708)