Label: POVI-ONE- povidone-iodine 10% topical liquid
- NDC Code(s): 57511-0611-2
- Packager: Elevate Oral Care
- This is a repackaged label.
- Source NDC Code(s): 68599-3500
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
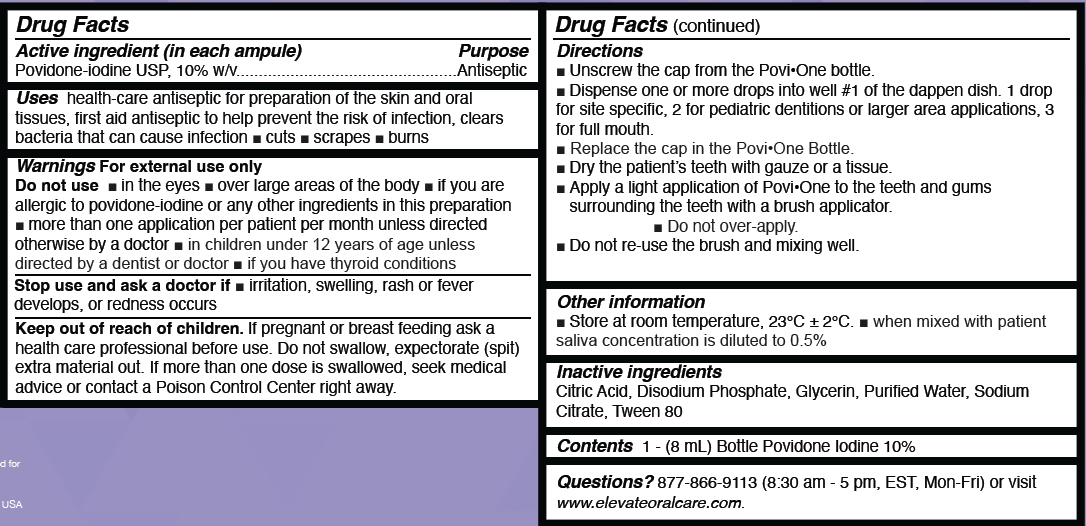
- Drug Facts Active Ingredients
- Uses Purpose
- Warnings For External Use Do Not Use
- Stop Use And Ask A Doctor
- Keep out of Reach of Children
- Directions
- Other Information
- Inactive Ingredients
- Contents
- Questions?
- Instructions for Use
- Dosage and Administration
- Povi-One Solution Outside Box label
-
INGREDIENTS AND APPEARANCE
POVI-ONE
povidone-iodine 10% topical liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57511-0611(NDC:68599-3500) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 1 mg in 10 mL Inactive Ingredients Ingredient Name Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) WATER (UNII: 059QF0KO0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color brown (Liquid) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57511-0611-2 8 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 12/18/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/13/2024 Labeler - Elevate Oral Care (002863526) Establishment Name Address ID/FEI Business Operations Elevate Oral Care 002863526 relabel(57511-0611) , repack(57511-0611)