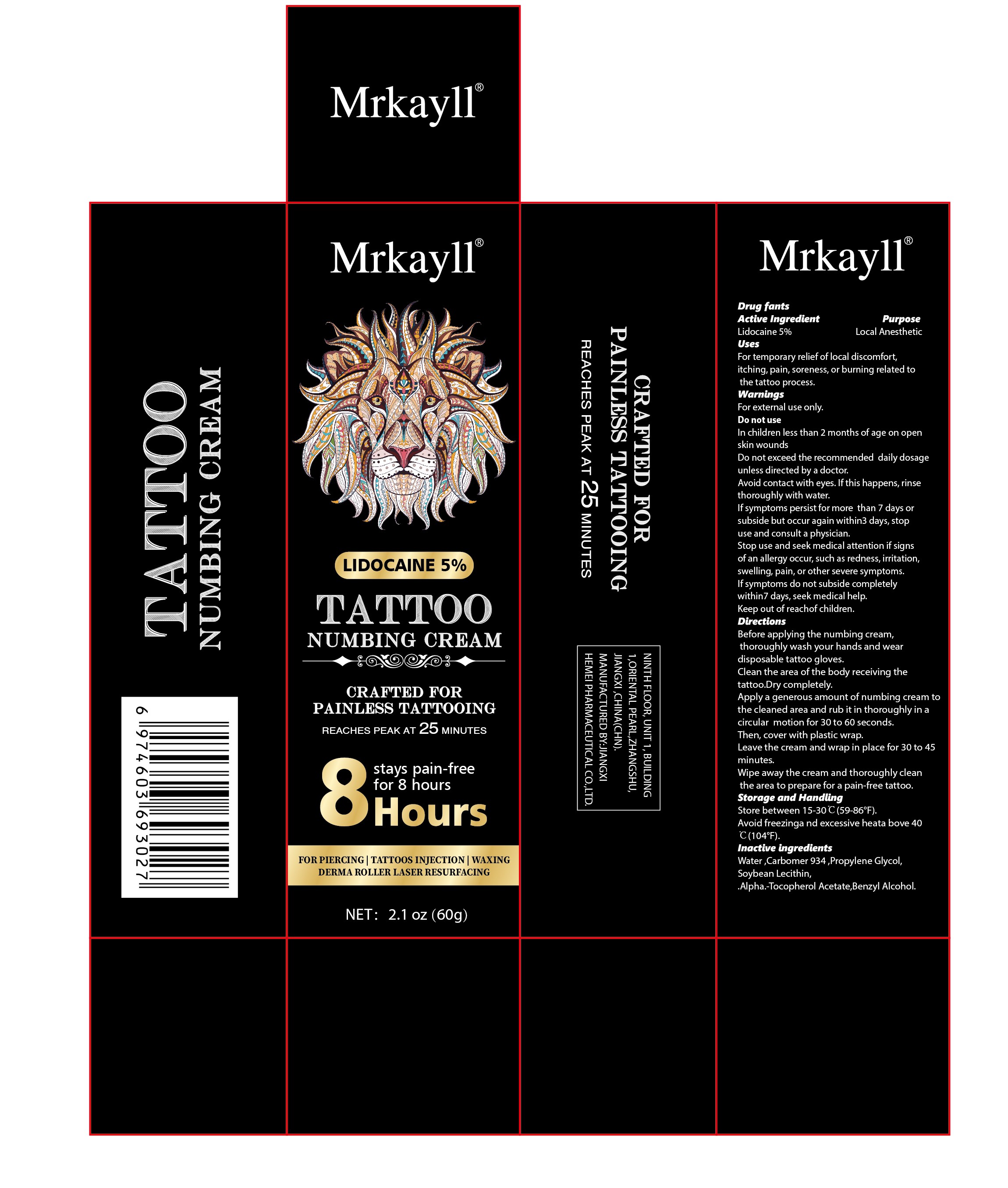
Label: MRKAYLL TATTOO NUMBING- lidocaine5% tattoo numbing cream
- NDC Code(s): 84010-045-01, 84010-045-02, 84010-045-03
- Packager: Jiangxi Hemei Pharmaceutical Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
-
Directions
Before applying the numbing cream, thoroughly wash your hands and wear disposable tattoo gloves.
Clean the area of the body receivina the tattoo,Dry completely.
Apply a generous amount of numbing cream to the cleaned area and rub it in thoroughly in a circlar motion for 30 to 60 seconds,Then. cover with plastic wrap.
Leave the cream and wrap in place for 30 to 45 minutes,Wipe away the cream and thoroughly clean the area to prepare for a pain-free tattoo.
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MRKAYLL TATTOO NUMBING
lidocaine5% tattoo numbing creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84010-045 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 5 g in 100 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER 934 (UNII: Z135WT9208) SOYBEAN LECITHIN (UNII: 1DI56QDM62) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84010-045-01 20 g in 1 TUBE; Type 0: Not a Combination Product 11/19/2024 2 NDC:84010-045-02 50 g in 1 TUBE; Type 0: Not a Combination Product 11/19/2024 3 NDC:84010-045-03 60 g in 1 TUBE; Type 0: Not a Combination Product 11/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/19/2024 Labeler - Jiangxi Hemei Pharmaceutical Co., Ltd (724892056) Establishment Name Address ID/FEI Business Operations Jiangxi Hemei Pharmaceutical Co., Ltd 724892056 manufacture(84010-045)