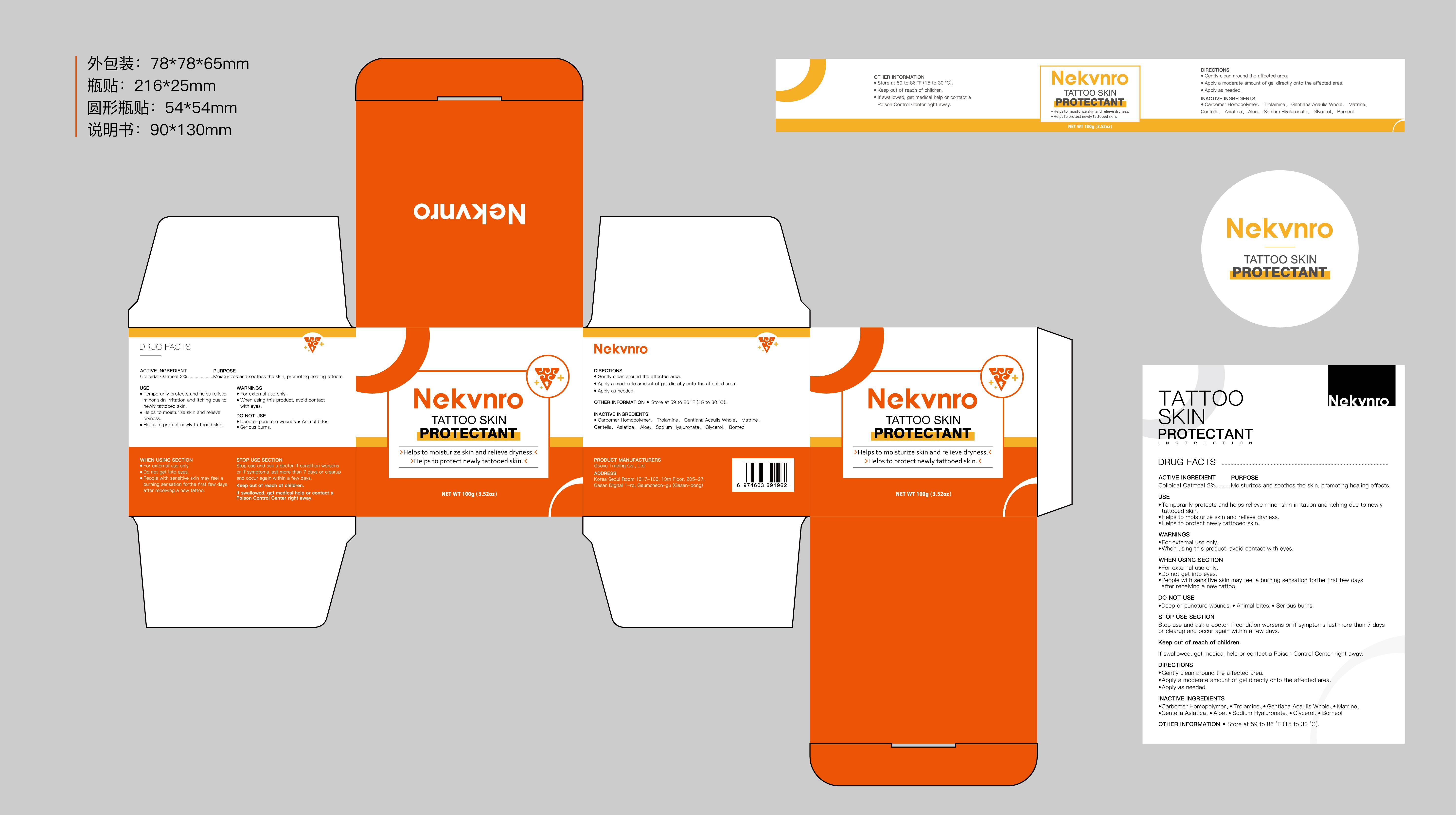
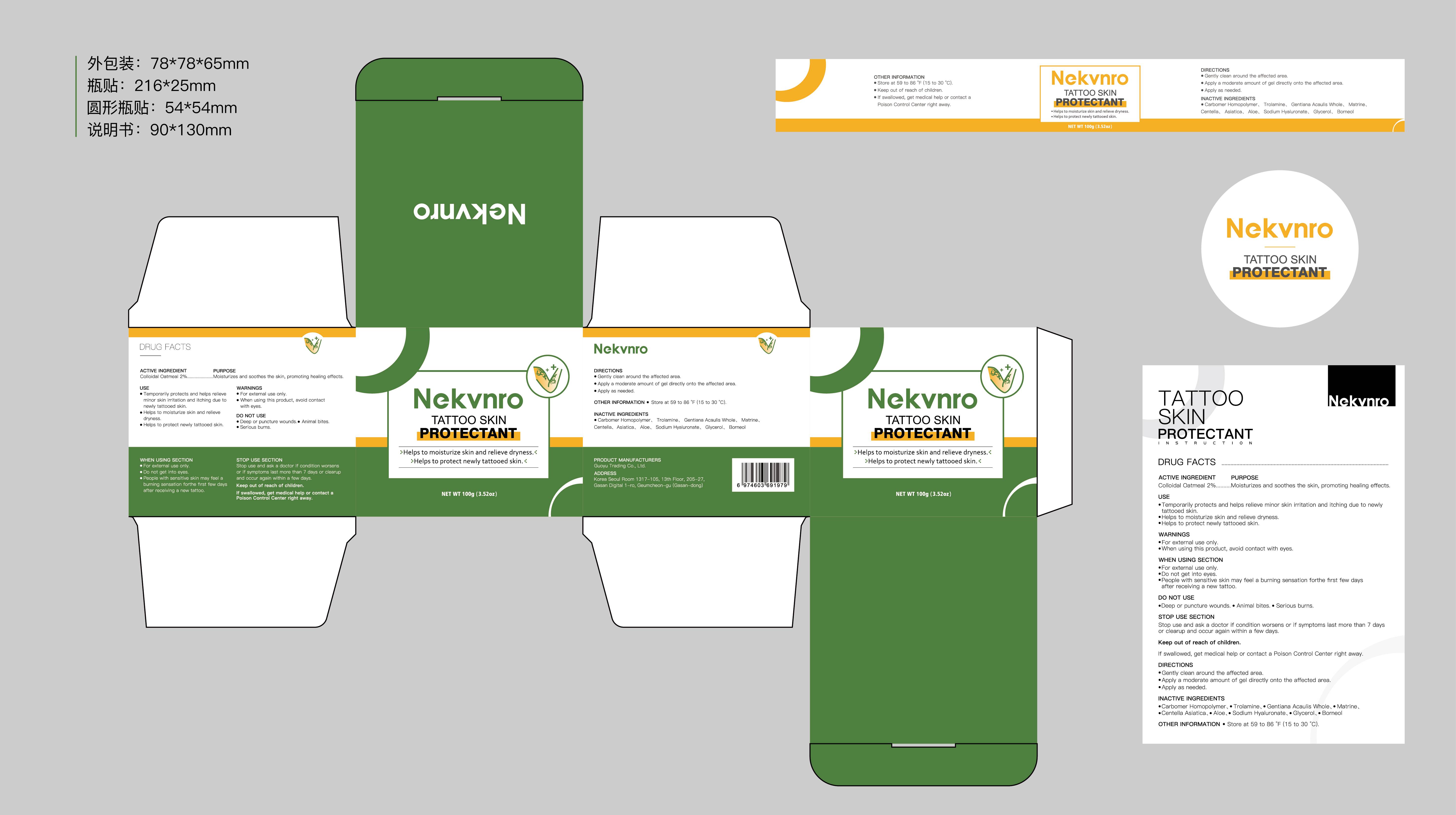
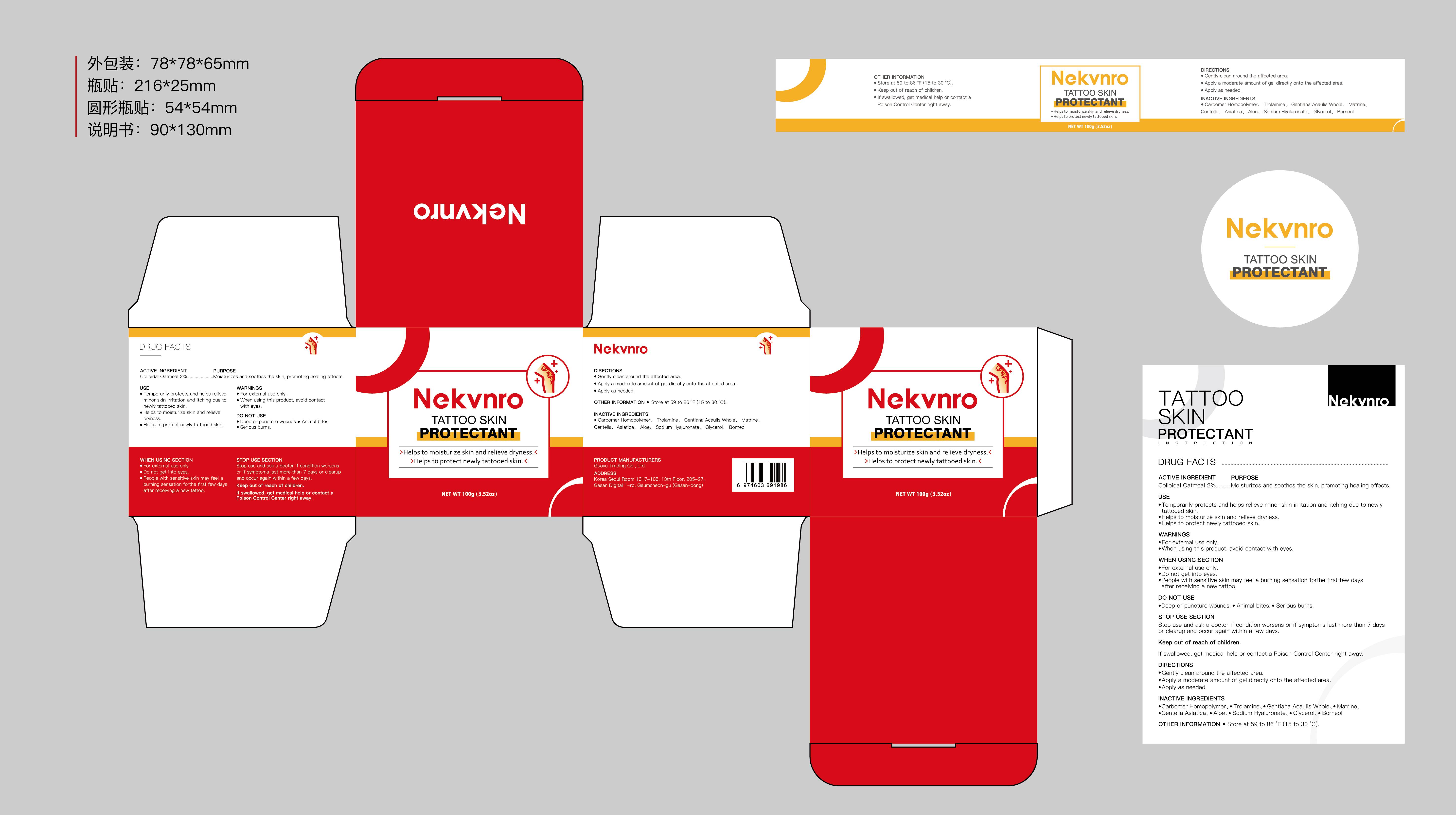
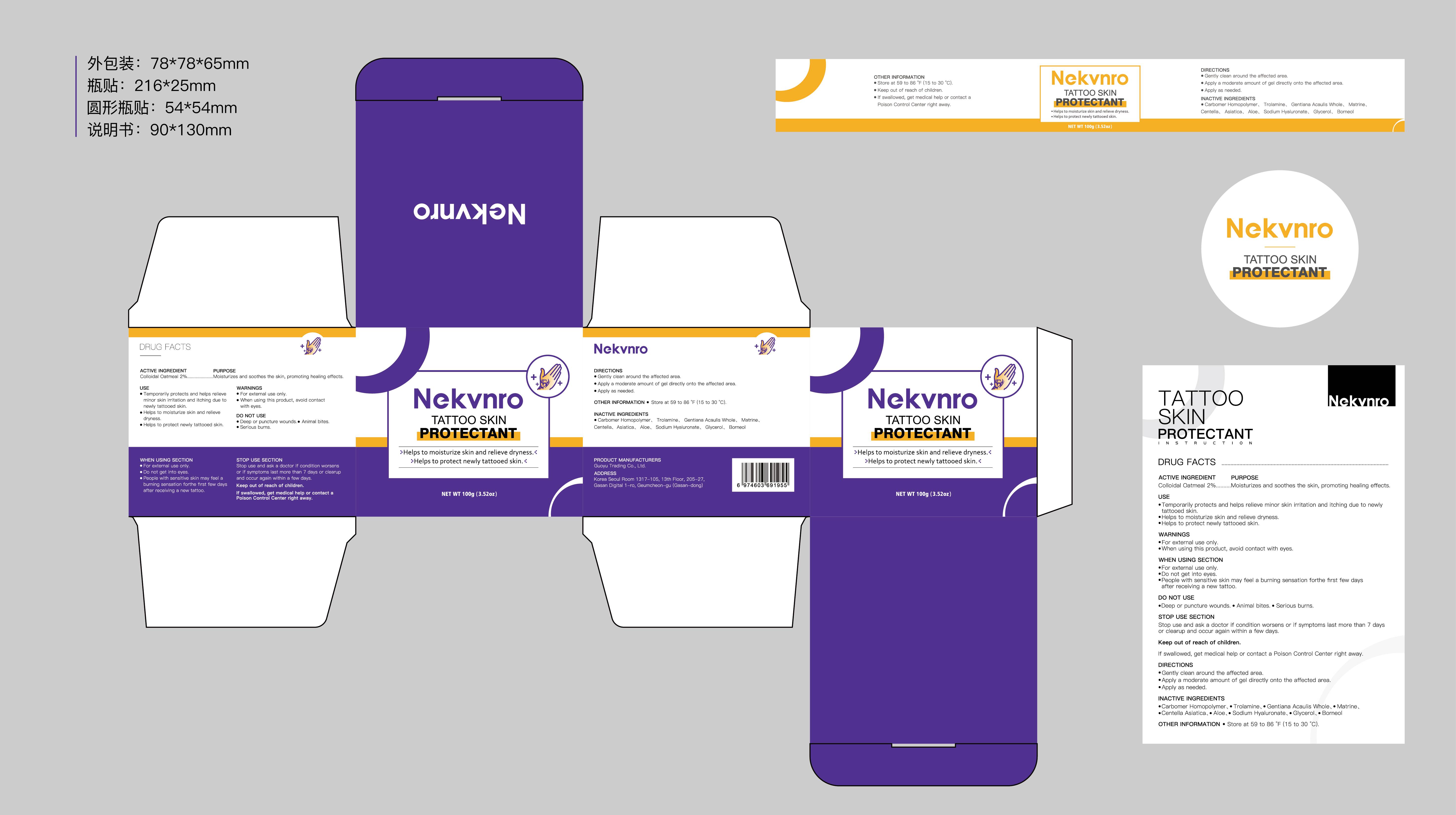
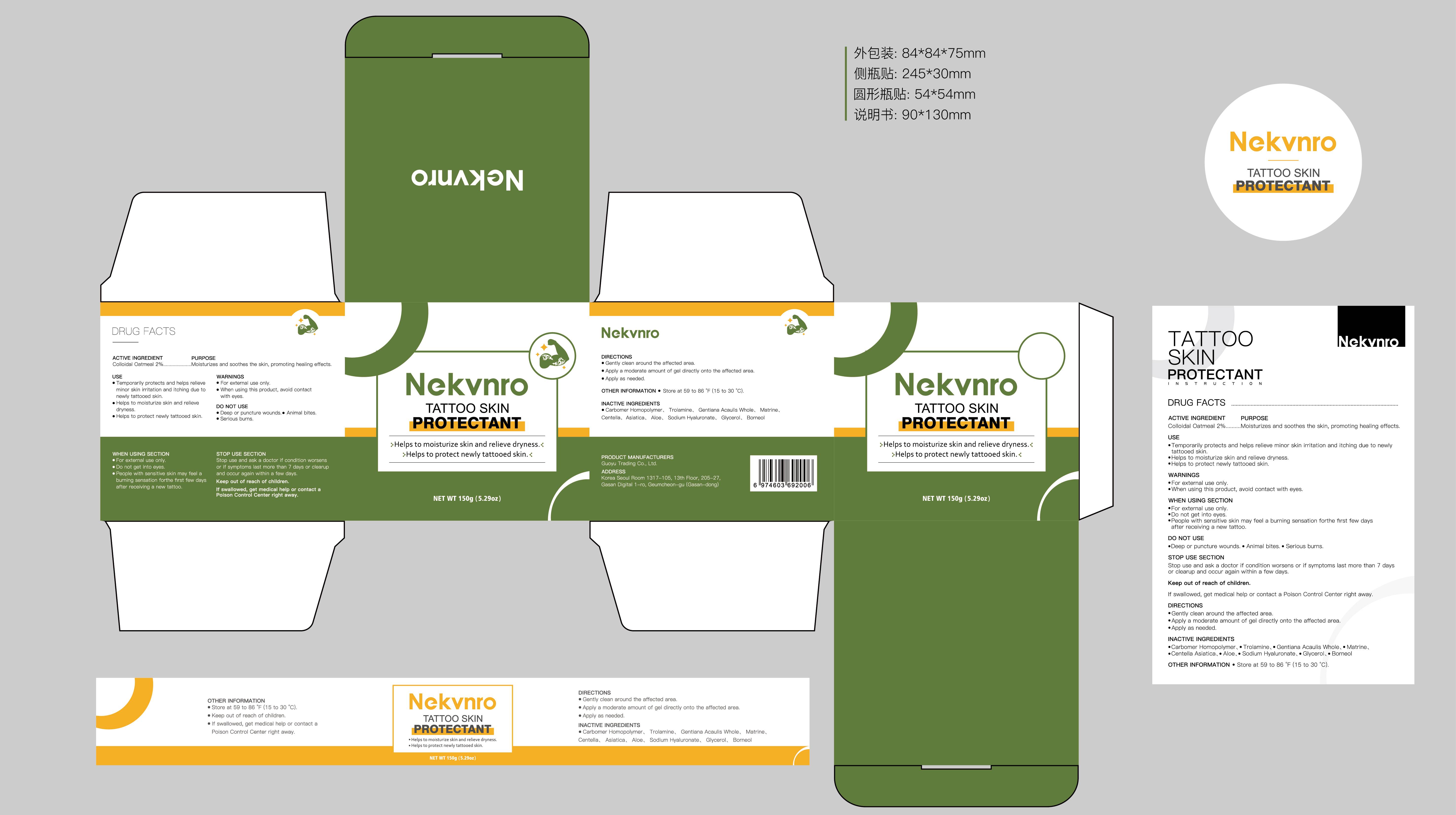
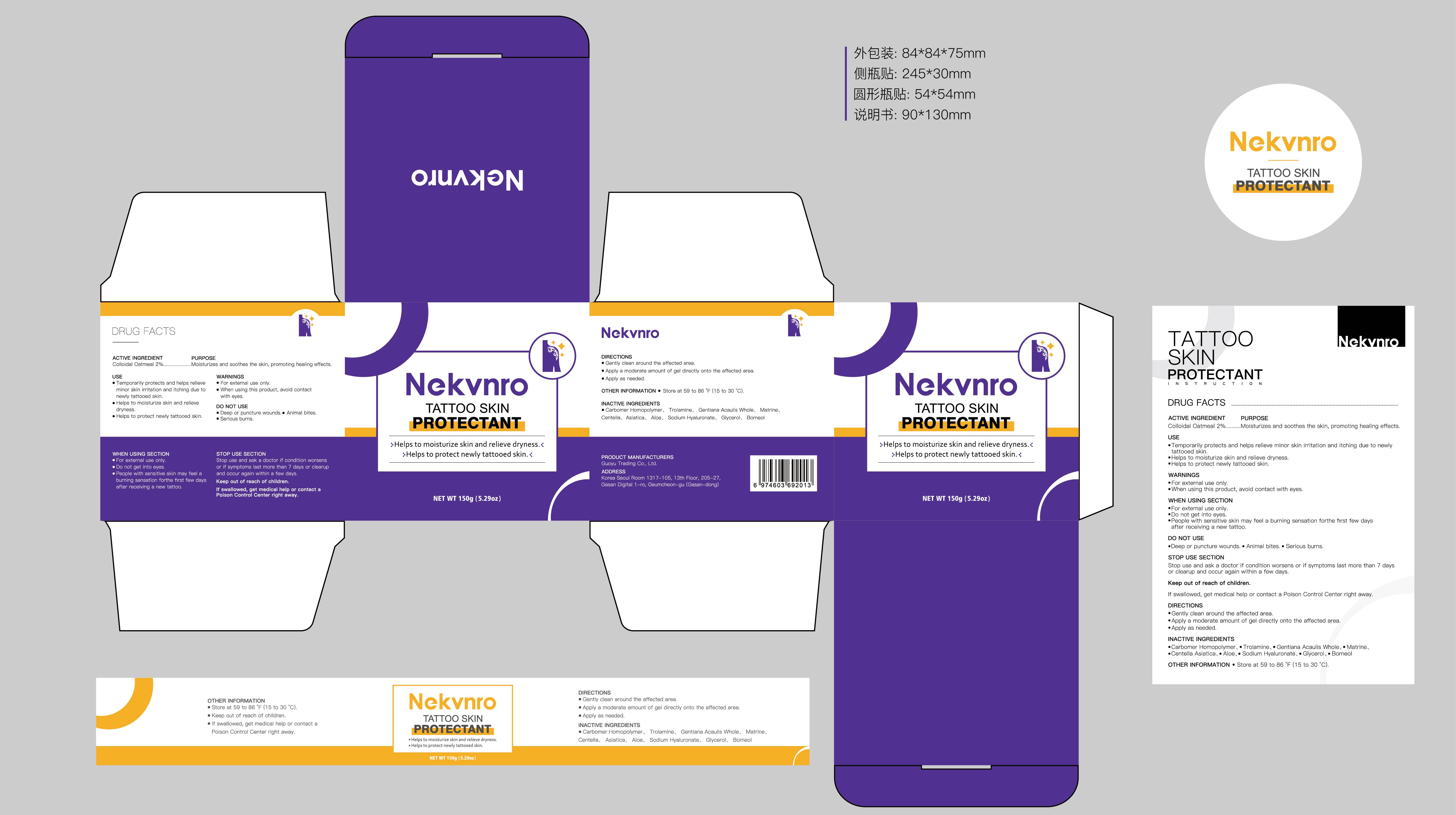
Label: NEKVNRO TATTOO SKIN PROTECTANT- tattoo skin protectant cream
- NDC Code(s): 84844-014-01, 84844-014-02
- Packager: Guoyu Trading Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEKVNRO TATTOO SKIN PROTECTANT
tattoo skin protectant creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84844-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COLLOIDAL OATMEAL EXTRACT (UNII: 8PI54V663Y) (COLLOIDAL OATMEAL EXTRACT - UNII:8PI54V663Y) COLLOIDAL OATMEAL EXTRACT 2 g in 100 g Inactive Ingredients Ingredient Name Strength CENTELLA ASIATICA (UNII: 7M867G6T1U) GLYCERIN (UNII: PDC6A3C0OX) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) MATRINE (UNII: N390W430AC) BORNEOL (UNII: M89NIB437X) TROLAMINE (UNII: 9O3K93S3TK) GENTIANA ACAULIS WHOLE (UNII: 0SR9G8J0FE) ALOE (UNII: V5VD430YW9) SODIUM HYALURONATE (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84844-014-01 100 g in 1 CAN; Type 0: Not a Combination Product 11/15/2024 2 NDC:84844-014-02 150 g in 1 CAN; Type 0: Not a Combination Product 11/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/15/2024 Labeler - Guoyu Trading Co., Ltd. (963348600) Establishment Name Address ID/FEI Business Operations Guoyu Trading Co., Ltd. 963348600 manufacture(84844-014)