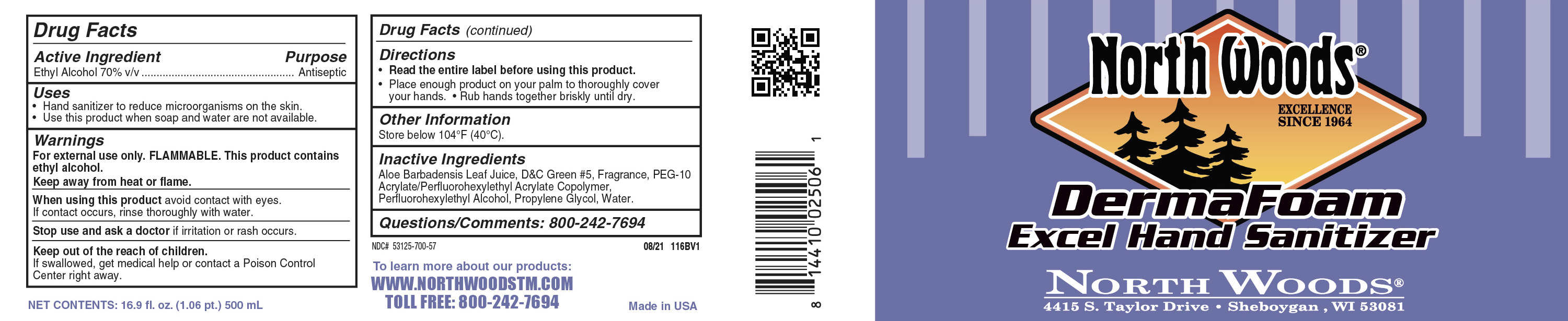
Label: ETHANOL- derma foam excel hand sanitizer soap
- NDC Code(s): 53125-803-88
- Packager: Superior Chemical Co DBA NorthWoods
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Warning statement
- purpose
- when using directions
- inactive ingredients
- keep out of reach of children wording
- Directions for usage and dosage
- indications and usage
- principle label
-
INGREDIENTS AND APPEARANCE
ETHANOL
derma foam excel hand sanitizer soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53125-803 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) 2-SEC-BUTYL CYCLOHEXANONE (UNII: 5WA6R1KL5J) WATER (UNII: 059QF0KO0R) 4-CYCLOHEXYL-2-METHYL-2-BUTANOL (UNII: HFL24LW6V5) ALOE VERA LEAF (UNII: ZY81Z83H0X) HEXYL ACETATE (UNII: 7U7KU3MWT0) TETRAHYDROLINALOOL (UNII: UM4XS5M134) ISOPROPYLPHENYLBUTANAL (UNII: Z92022479Y) 2-(PERFLUOROHEXYL)ETHANETHIOL (UNII: 66E2ZQ1P07) PEG-10 ACRYLATE/PERFLUOROHEXYLETHYL ACRYLATE COPOLYMER (UNII: D76Z87928N) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53125-803-88 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 11/14/2024 Labeler - Superior Chemical Co DBA NorthWoods (023335086) Registrant - Betco Corp LLC (005050158)