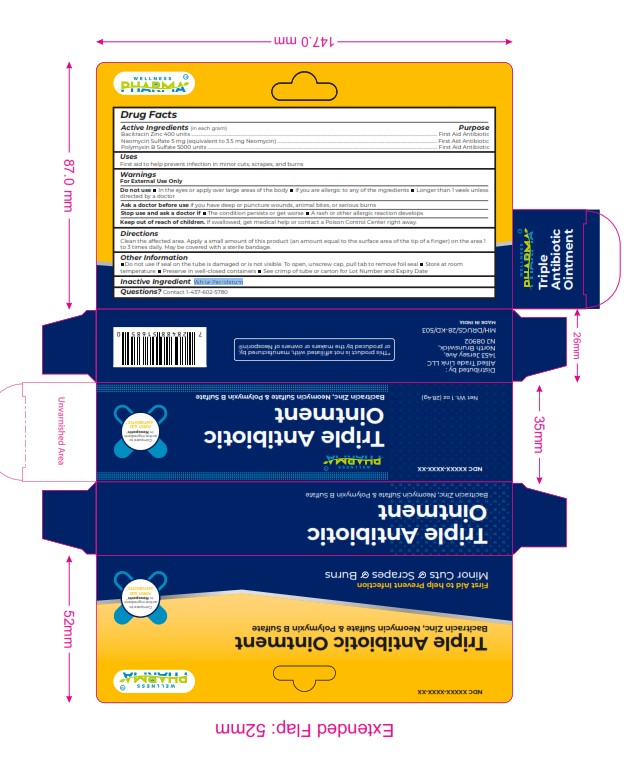
Label: TRIPLET ANTIBIOTIC ointment
- NDC Code(s): 84387-006-01
- Packager: ALLIED TRADE LINK LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DOSAGE & ADMINISTRATION
Active Ingredients (in each gram) Purpose
Bacitracin Zinc 400 units ................................................................................................................................................................................................................... First Aid Antibiotic
Neomycin Sulfate 5 mg (equivalent to 3.5 mg Neomycin) ......................................................................................................................................... First Aid Antibiotic
Polymyxin B Sulfate 5000 units ..................................................................................................................................................................................................... First Aid Antibiotic -
WARNINGS
For External Use Only
Do not use In the eyes or apply over large areas of the body If you are allergic to any of the ingredients Longer than 1 w eek unless
directed by a doctor
Ask a doctor before use if you have deep or puncture wounds, animal bites, or serious burns
Stop use and ask a doctor if The condition persists or get worse A rash or other allergic reaction develops
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRIPLET ANTIBIOTIC
triplet antibiotic ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84387-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [USP'U] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g Inactive Ingredients Ingredient Name Strength WHITE PETROLATUM (UNII: B6E5W8RQJ4) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84387-006-01 28.4 g in 1 TUBE; Type 0: Not a Combination Product 11/12/2024 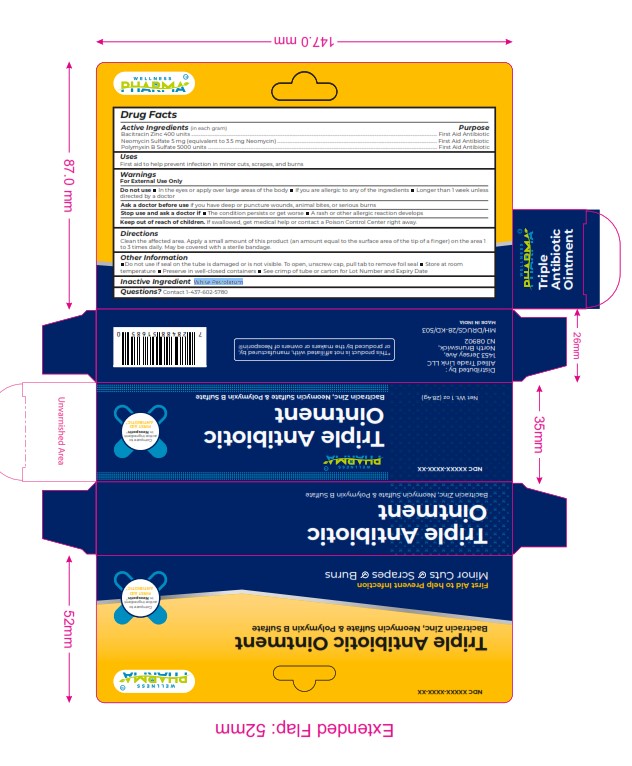
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 11/12/2024 Labeler - ALLIED TRADE LINK LLC (029033682) Registrant - ALLIED TRADE LINK LLC (029033682) Establishment Name Address ID/FEI Business Operations GOLPALDAS VISRAM AND COMPANY LIMITED 858030888 manufacture(84387-006)