Label: METOPROLOL SUCCINATE- metoprolol succinate er tablets tablet, film coated, extended release
- NDC Code(s): 67046-1109-3
- Packager: Coupler LLC
- This is a repackaged label.
- Source NDC Code(s): 68001-502
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METOPROLOL SUCCINATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for METOPROLOL SUCCINATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGE1.1 Hypertension - Metoprolol succinate extended-release tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and ...
-
2 DOSAGE & ADMINISTRATION2.1 Hypertension - Adults:The usual initial dosage is 25 mg to 100 mg daily in a single dose. Adjust dosage at weekly (or longer) intervals until optimum blood pressure reduction is achieved. In ...
-
3 DOSAGE FORMS & STRENGTHSMetoprolol Succinate Extended-Release Tablets, USP are available containing 23.75 mg, 47.5 mg, 95 mg or 190mg of metoprolol succinate, USP equivalent to 25 mg, 50 mg, 100 mg or 200 mg of ...
-
4 CONTRAINDICATIONSMetoprolol succinate extended-release tablets are contraindicated in severe bradycardia, second- or third-degree heart block, cardiogenic shock, decompensated heart failure, sick sinus syndrome ...
-
5 WARNINGS AND PRECAUTIONS5.1 Abrupt Cessation of Therapy - Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in labeling: Worsening angina or myocardial infarction [see Warnings and Precautions ( 5)]. Worsening heart failure [see ...
-
7 DRUG INTERACTIONS7.1 Catecholamine Depleting Drugs - Catecholamine depleting drugs (e.g., reserpine, monoamine oxidase (MAO) inhibitors) may have an additive effect when given with beta-blocking agents. Observe ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Untreated hypertension and heart failure during pregnancy can lead to adverse outcomes for the mother and the fetus - (see Clinical Considerations).Available data ...
-
10 OVERDOSAGESigns and Symptoms - Overdosage of metoprolol succinate extended-release tablets may lead to severe bradycardia, hypotension, and cardiogenic shock. Clinical presentation can also include ...
-
11 DESCRIPTIONMetoprolol succinate, is a beta - 1-selective (cardioselective) adrenoceptor blocking agent, for oral administration, available as extended-release tablets. Metoprolol succinate ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metoprolol is a beta - 1-selective (cardioselective) adrenergic receptor blocking agent. This preferential effect is not absolute, however, and at higher plasma ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - Long-term studies in animals have been conducted to evaluate the carcinogenic potential of metoprolol tartrate. In 2-year studies in ...
-
14 CLINICAL STUDIES14.1 Hypertension - In a double-blind study, 1092 patients with mild-to-moderate hypertension were randomized to once daily metoprolol succinate extended-release tablets (25 mg, 100 mg, or 400 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMetoprolol Succinate Extended-Release Tablets, USP are available containing 23.75 mg, 47.5 mg, 95 mg or 190 mg of metoprolol succinate, USP equivalent to 25 mg, 50 mg, 100 mg or 200 mg of ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to take metoprolol succinate extended-release tablets regularly and continuously, as directed, preferably with or immediately following meals. If a dose is missed, the patient ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 68001-500-00 - 25mg - 100 Tablets - Rx Only
-
Package/Label Display Panel
NDC 68001-501-00 - 50mg - 100 Tablets - Rx Only
-
Package/Label Display Panel
NDC 68001-502-00 - 100mg - 100 Tablets - Rx Only
-
Package/Label Display Panel
NDC 68001-503-00 - 200mg - 100 Tablets - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information





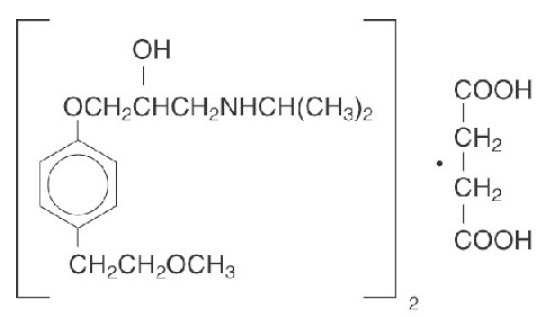 Metoprolol succinate, is a beta
Metoprolol succinate, is a beta
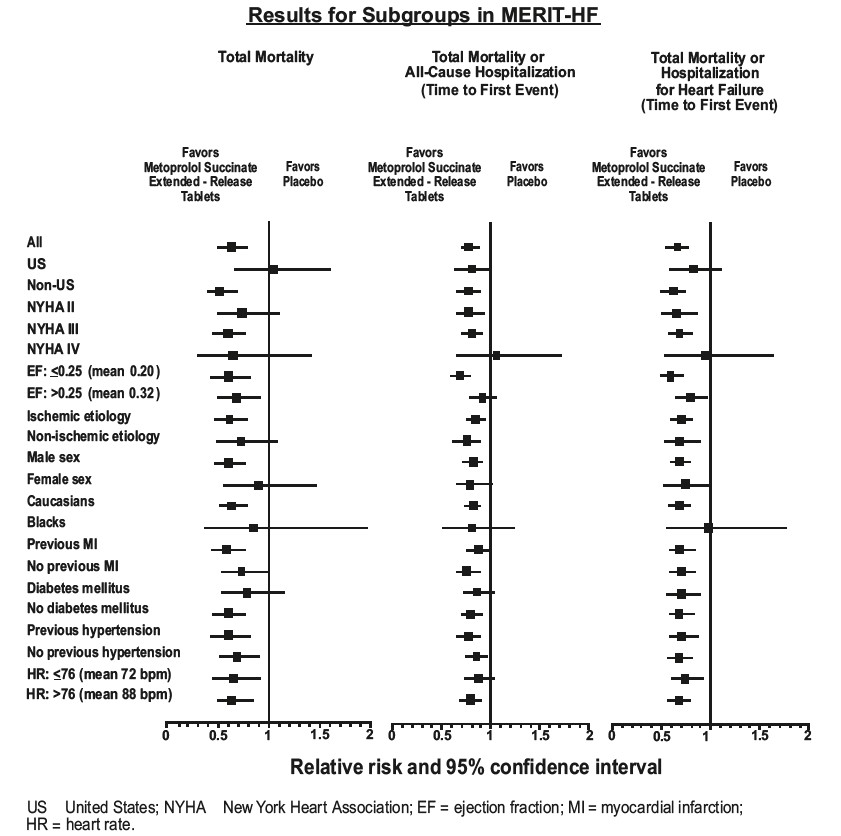 MERIT-HF was a randomized double-blind, placebo-controlled study of metoprolol succinate extended-release tablets in which 3991 patients with ejection fraction ≤0.40 and NYHA Class II-IV heart failure attributable to ischemia, hypertension, or cardiomyopathy were randomized 1:1 to metoprolol succinate extended-release tablets or placebo. The protocol excluded patients with contraindications to betablocker use, those expected to undergo heart surgery, and those within 28 days of myocardial infarction or unstable angina. The primary endpoints of the trial were (1) all-cause mortality plus all-cause hospitalization (time to first event) and (2) all-cause mortality. Patients were stabilized on optimal concomitant therapy for heart failure, including diuretics, ACE inhibitors, cardiac glycosides, and nitrates. At randomization, 41% of patients were NYHA Class II; 55% NYHA Class III; 65% of patients had heart failure attributed to ischemic heart disease; 44% had a history of hypertension; 25% had diabetes mellitus; 48% had a history of myocardial infarction. Among patients in the trial, 90% were on diuretics, 89% were on ACE inhibitors, 64% were on digitalis, 27% were on a lipid-lowering agent, 37% were on an oral anticoagulant, and the mean ejection fraction was 0.28. The mean duration of follow-up was one year. At the end of the study, the mean daily dose of metoprolol succinate extended-release tablets were 159 mg.
MERIT-HF was a randomized double-blind, placebo-controlled study of metoprolol succinate extended-release tablets in which 3991 patients with ejection fraction ≤0.40 and NYHA Class II-IV heart failure attributable to ischemia, hypertension, or cardiomyopathy were randomized 1:1 to metoprolol succinate extended-release tablets or placebo. The protocol excluded patients with contraindications to betablocker use, those expected to undergo heart surgery, and those within 28 days of myocardial infarction or unstable angina. The primary endpoints of the trial were (1) all-cause mortality plus all-cause hospitalization (time to first event) and (2) all-cause mortality. Patients were stabilized on optimal concomitant therapy for heart failure, including diuretics, ACE inhibitors, cardiac glycosides, and nitrates. At randomization, 41% of patients were NYHA Class II; 55% NYHA Class III; 65% of patients had heart failure attributed to ischemic heart disease; 44% had a history of hypertension; 25% had diabetes mellitus; 48% had a history of myocardial infarction. Among patients in the trial, 90% were on diuretics, 89% were on ACE inhibitors, 64% were on digitalis, 27% were on a lipid-lowering agent, 37% were on an oral anticoagulant, and the mean ejection fraction was 0.28. The mean duration of follow-up was one year. At the end of the study, the mean daily dose of metoprolol succinate extended-release tablets were 159 mg.
 NDC 68001-500-00
NDC 68001-500-00
 NDC 68001-501-00
NDC 68001-501-00
 NDC 68001-502-00
NDC 68001-502-00
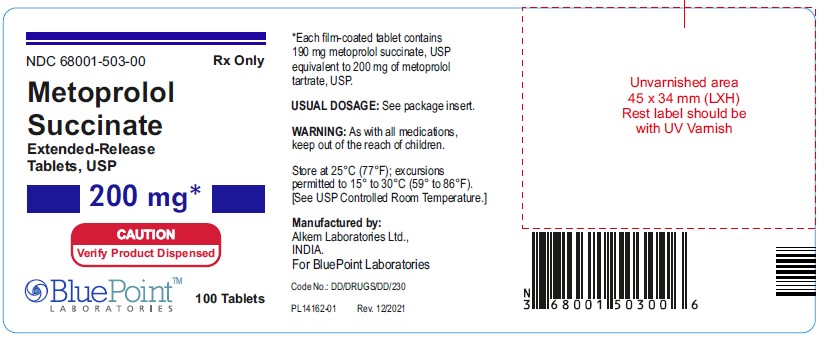 NDC 68001-503-00
NDC 68001-503-00