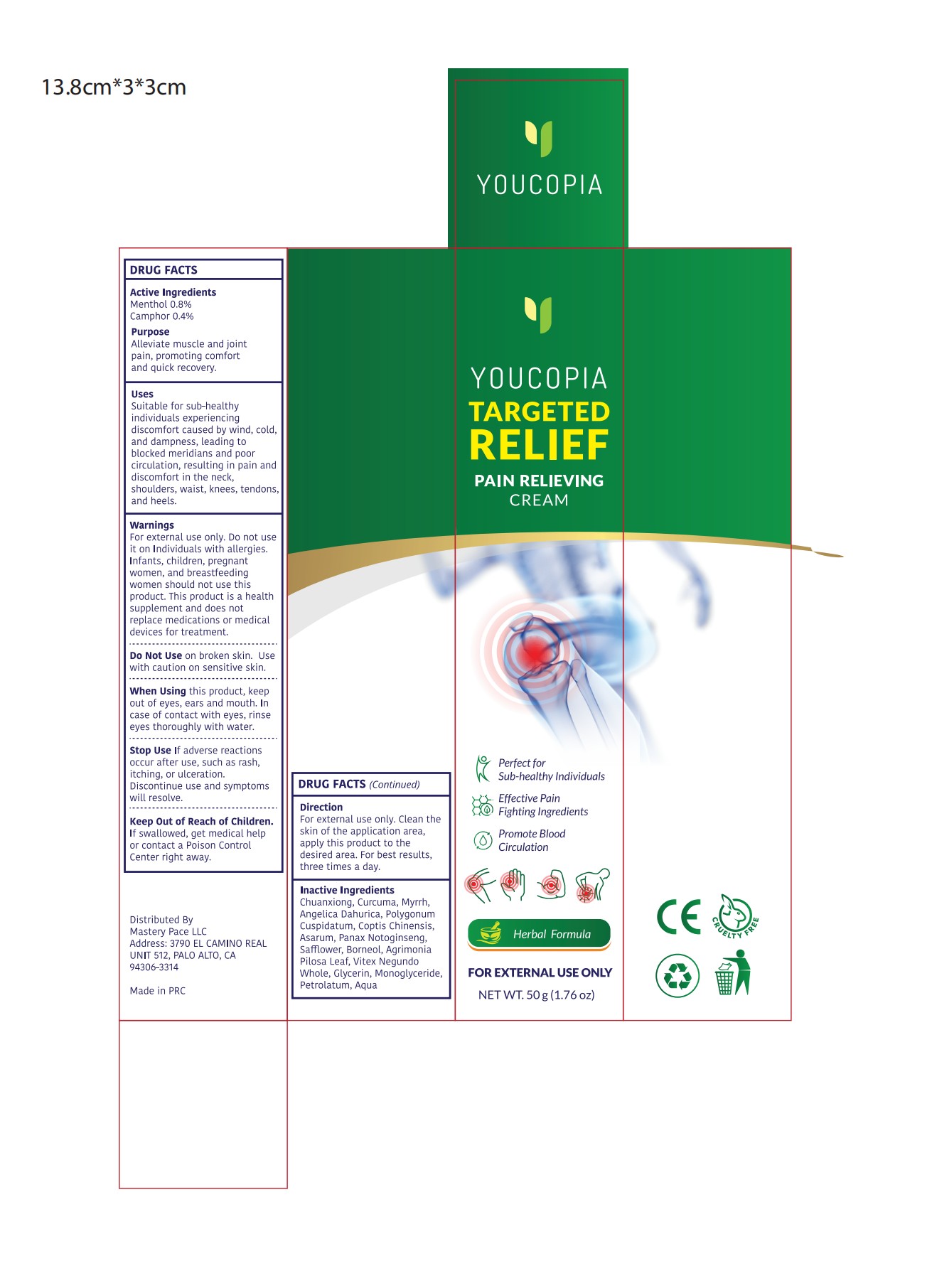
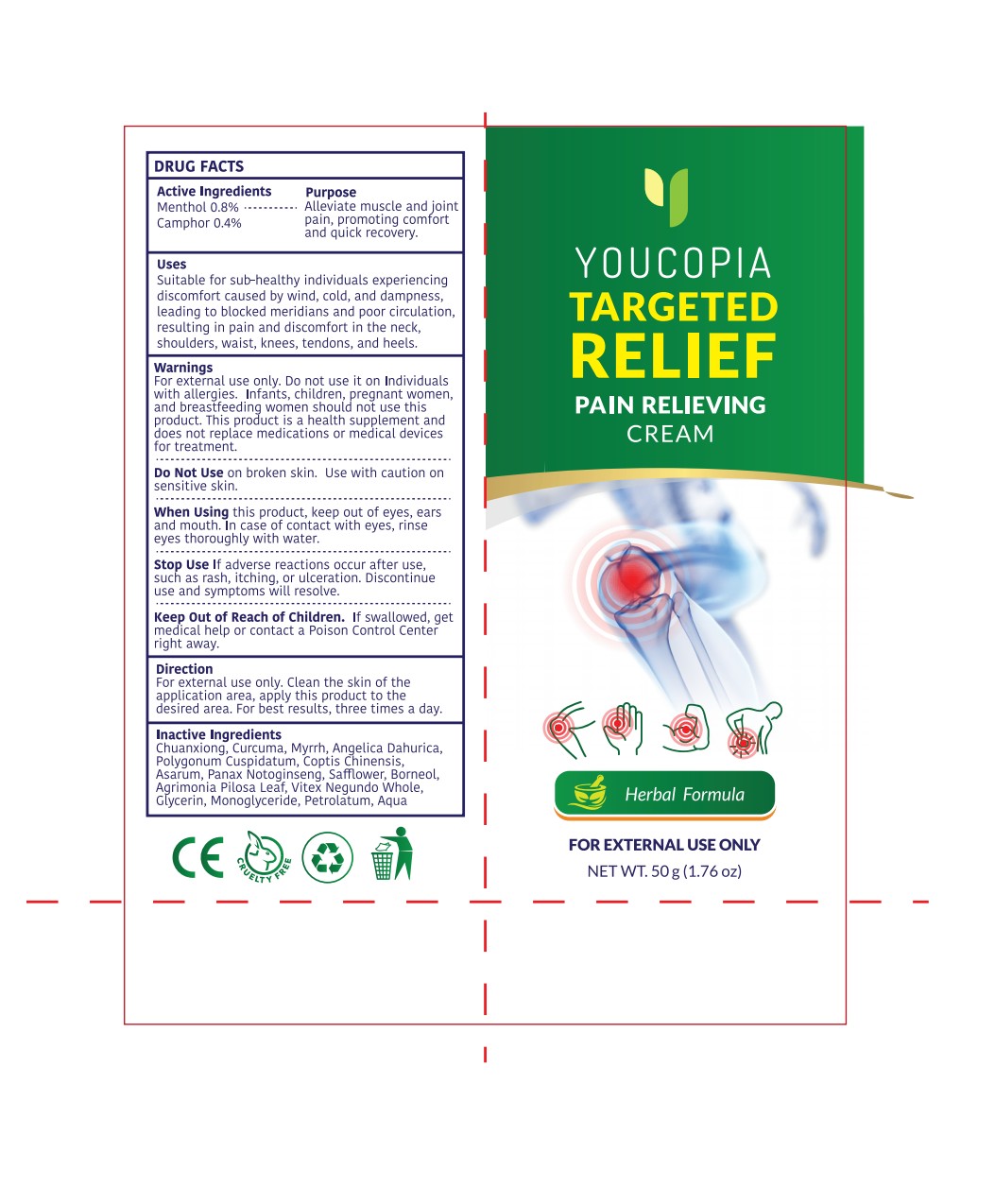
Label: YOUCOPIA PAIN RELIEF CREAM- pain relief cream cream
- NDC Code(s): 82372-014-01
- Packager: Good Manager Holdings Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
YOUCOPIA PAIN RELIEF CREAM
pain relief cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82372-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.8 g in 100 g CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 0.4 g in 100 g Inactive Ingredients Ingredient Name Strength POLYGONUM CUSPIDATUM LEAF (UNII: 2540B7G25G) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) BORNEOL (UNII: M89NIB437X) PETROLATUM (UNII: 4T6H12BN9U) COPTIS CHINENSIS ROOT (UNII: CXS4LJR7EL) AGRIMONIA PILOSA LEAF (UNII: 6E03WRR337) CURCUMA LONGA (TURMERIC) RHIZOME EXTRACT (UNII: 856YO1Z64F) VITEX NEGUNDO WHOLE (UNII: C92PGK11XB) LIGUSTICUM CHUANXIONG ROOT EXTRACT (UNII: RR83T99U97) MYRRH (UNII: JC71GJ1F3L) ASARUM CAUDATUM ROOT (UNII: 93NNE1H1O7) PANAX NOTOGINSENG ROOT (UNII: GQX1C1175U) SAFFLOWER (UNII: 4VBL71TY4Y) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82372-014-01 50 g in 1 TUBE; Type 0: Not a Combination Product 11/12/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/12/2024 Labeler - Good Manager Holdings Inc. (118382673) Establishment Name Address ID/FEI Business Operations Good Manager Holdings Inc. 118382673 manufacture(82372-014)