Label: VISTOGARD- uridine triacetate granule
- NDC Code(s): 50633-220-04, 50633-220-20
- Packager: BTG International Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VISTOGARD safely and effectively. See full prescribing information for VISTOGARD. VISTOGARD - ®(uridine triacetate) oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEVISTOGARD - ®is indicated for the emergency treatment of adult and pediatric patients: following a fluorouracil or capecitabine overdose regardless of the presence of symptoms, or - who ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Adults: 10 grams (1 packet) orally every 6 hours for 20 doses, without regard to meals. Pediatric: 6.2 grams/m - 2of body surface area (not to exceed 10 grams ...
-
3 DOSAGE FORMS AND STRENGTHSOral granules: 10 grams of orange-flavored, white-to-off-white, oral granules (95% w/w) in single-dose packets.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONSNone.
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions and using a wide range of doses, adverse reaction rates observed in the clinical trials of a ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited case reports of uridine triacetate use during pregnancy are insufficient to inform a drug-associated risk of birth defects and miscarriage. When ...
-
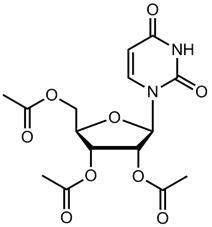
11 DESCRIPTIONVISTOGARD oral granules contain the active ingredient uridine triacetate which is a pyrimidine analog. The chemical name for uridine triacetate is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Uridine triacetate is an acetylated pro-drug of uridine. Following oral administration, uridine triacetate is deacetylated by nonspecific esterases present throughout ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate the carcinogenic potential of uridine triacetate. Uridine triacetate ...
-
14 CLINICAL STUDIESThe efficacy of VISTOGARD was assessed in 135 patients who were treated in two open-label trials, Study 1 (n=60) and Study 2 (n=75). The patients in both studies had either received an overdose of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGVISTOGARD orange-flavored oral granules (95% w/w) are available in single-dose packets containing 10 grams of uridine triacetate. NDC NumberPackage Configuration - 50633-220-20Course of ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient or caregiver to read the FDA-approved patient labeling (Patient Information) Dosing Instructions - [see - Dosage and Administration (2.1)and - (2.2) ...
-
SPL UNCLASSIFIED SECTIONManufactured for and distributed by: BTG International Inc. West Conshohocken, PA, 19428 - BTG - Pharmaceuticals - a SERB company - VISTOGARD - ®is a registered trademark of BTG ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug AdministrationIssued: 10/2023 - Patient Information - VISTOGARD (VIS-toe-gard) (uridine triacetate ...
-
PRINCIPAL DISPLAY PANEL - 20 Count - 10 g Packet CartonNDC 50633-220-20 - Rx Only - VISTOGARD - ®10 g - (uridine triacetate) oral granules - Carton contains 20 x 10 gram packets - BTG - Pharmaceuticals
-
PRINCIPAL DISPLAY PANEL - 4 Count 10 g Packet CartonNDC 50633-220-04 - Rx Only - VISTOGARD - ®10 g - (uridine triacetate) oral granules - Carton contains 4 x 10 gram packets - BTG - Pharmaceuticals
-
PRINCIPAL DISPLAY PANEL - 10 g Single Dose Sachet FoilNDC 50633-220-10 - Rx Only - VISTOGARD - ®10 g - (uridine triacetate) oral granules - Single-Dose Packets - BTG - Pharmaceuticals
-
INGREDIENTS AND APPEARANCEProduct Information