Label: DENTEK CANKER COVER- menthol patch, extended release
- NDC Code(s): 10564-0910-2
- Packager: Bershtel Enterprises
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Keep Out of Reach of Children Section
- Purpose Section
- Ask Doctor
-
Indications & Usage
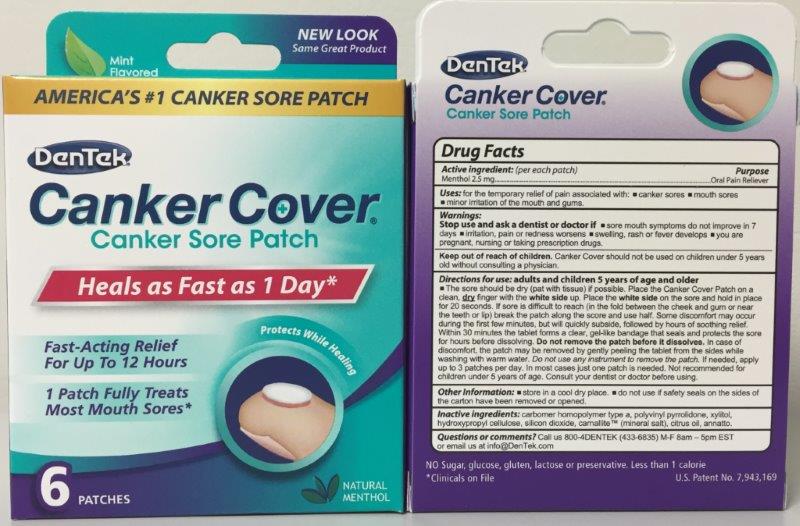
Adult and Children 5 year of age and older.
The sore should be dry (pat with tissue) is possible. Place the Canker Cover Patch on a clean, dry finger with the white side up. Place the white side on the sore and hold in place for 20 seconds. Within 30 minutes the tablet forms a clear, gel-like bandage that seals and protects the sore for hours before disolving. DO NOT REMOVE PATCH BEFORE IT DISSOLVES.
- Dosage & Administration
- Questions section
- Warning Section
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DENTEK CANKER COVER
menthol patch, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10564-0910 Route of Administration TRANSMUCOSAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2.5 mg in 194.85 Inactive Ingredients Ingredient Name Strength CITRUS MEDICA FRUIT (UNII: ZE5Q6PN9ON) CARBOMER 934 (UNII: Z135WT9208) POVIDONE (UNII: FZ989GH94E) HYDROXYPROPYL CELLULOSE (TYPE E) (UNII: 66O7AQV0RT) SEA SALT (UNII: 87GE52P74G) XYLITOL (UNII: VCQ006KQ1E) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color Score Shape ROUND Size 9mm Flavor MENTHOL Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10564-0910-2 1 in 1 CARTON; Type 0: Not a Combination Product 12/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 12/01/2015 Labeler - Bershtel Enterprises (066659129)