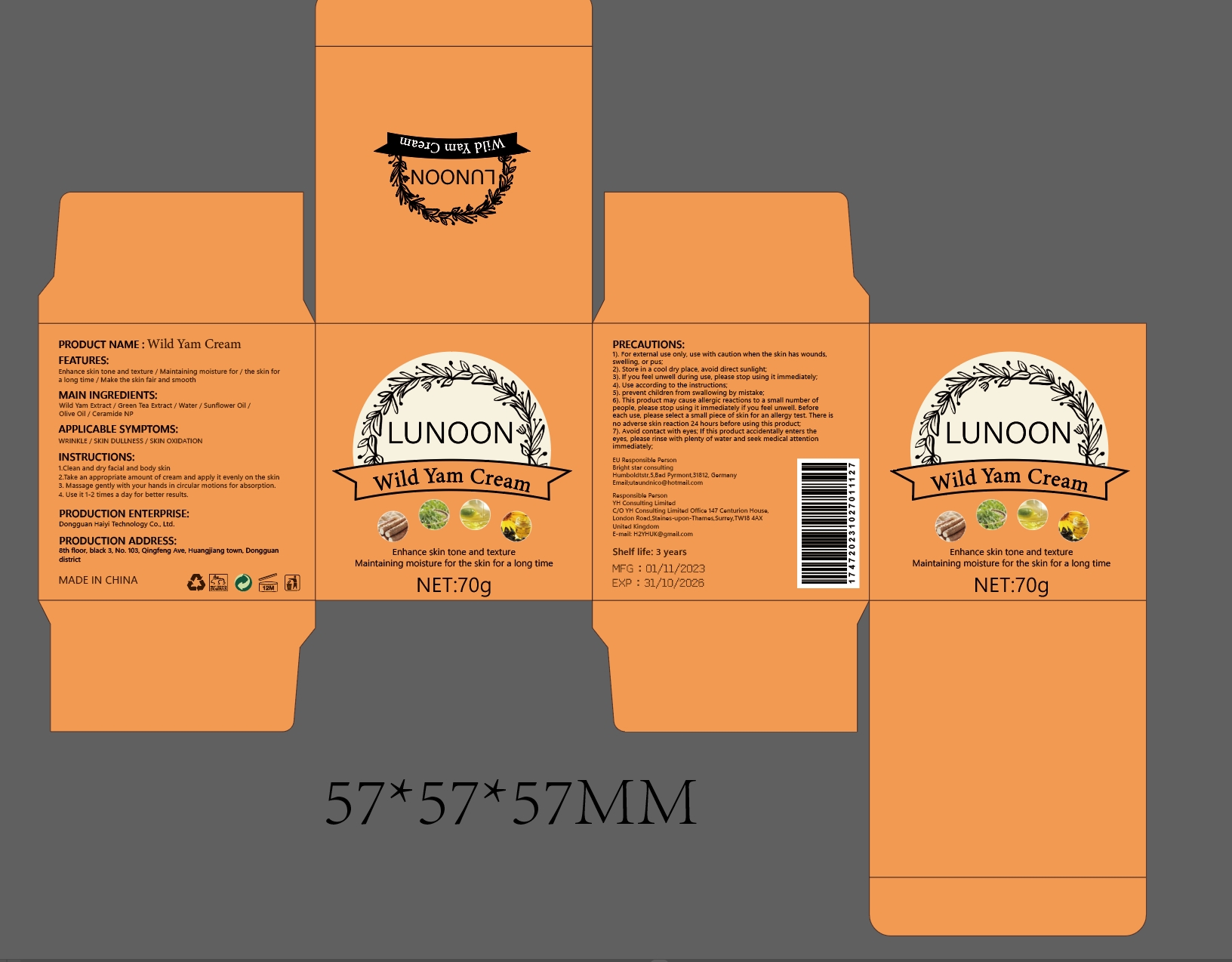
Label: WILD YAM CREAM cream
- NDC Code(s): 84732-043-02
- Packager: Dongguan Haiyi Technology Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Inactive ingredient
- Purpose section
-
Warning
1). For external use only, use with caution when the skin has wounds.swelling. or pus;
2). Store in a cool dry place, avoid direct sunlight
3). lf you feel unwell during use, please stop using it immediately.
4).Use according to the instructions;
5). prevent children from swallowing by mistake;
6). This product may cause allergic reactions to a small number ofpeople, please stop using it immediately if you feel unwell. Beforeeach use, please select a small piece of skin for an allergy test There isno adverse skin reaction 24 hours before using this product
7). Avoid contact with eyes, lf this product accidentally enters theeyes, please rinse with plenty of water and seek medical attentionimmediately.
- stop use
- not use
- OUT OF CHILDREN
- HOW TO USE
- Dosage
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WILD YAM CREAM
wild yam cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84732-043 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIOSCOREA VILLOSA TUBER (UNII: IWY3IWX2G8) (DIOSCOREA VILLOSA TUBER - UNII:IWY3IWX2G8) DIOSCOREA VILLOSA TUBER 1 mg in 100 g Inactive Ingredients Ingredient Name Strength GREEN TEA LEAF (UNII: W2ZU1RY8B0) CERAMIDE NP (UNII: 4370DF050B) WATER (UNII: 059QF0KO0R) SUNFLOWER OIL (UNII: 3W1JG795YI) OLIVE OIL (UNII: 6UYK2W1W1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84732-043-02 70 g in 1 BOTTLE; Type 0: Not a Combination Product 11/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/13/2024 Labeler - Dongguan Haiyi Technology Co.,Ltd. (722030807) Establishment Name Address ID/FEI Business Operations Dongguan Haiyi Technology Co.,Ltd. 722030807 manufacture(84732-043)