Label: BUDESONIDE capsule
- NDC Code(s): 51407-591-01
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0574-9855
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONBUDESONIDE DELAYED-RELEASE CAPSULES. These highlights do not include all the information needed to use - BUDESONIDE DELAYED-RELEASE CAPSULESsafely and effectively. See full prescribing information ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Treatment of Mild to Moderate Active Crohn’s Disease - Budesonide delayed-release capsules are indicated for the treatment of mild to moderate active Crohn's disease involving the ileum ...
-
2 DOSAGE AND ADMINISTRATION2.1 Administration Instructions - Take budesonide delayed-release capsules once daily in the morning. Swallow budesonide delayed-release capsules whole. Do not chew or crush. For patients unable ...
-
3 DOSAGE FORMS AND STRENGTHSDelayed-release Capsules: 3 mg hard gelatin capsules with an opaque light grey body and an opaque pink cap, coded with ENTOCORT EC 3 mg.
-
4 CONTRAINDICATIONSBudesonide delayed-release capsules are contraindicated in patients with hypersensitivity to budesonide or any of the ingredients of budesonide delayed-release capsules. Serious hypersensitivity ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypercorticism and Adrenal Axis Suppression - When corticosteroids are used chronically, systemic effects such as hypercorticism and adrenal axis suppression may occur. Corticosteroids can ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: Hypercorticism and adrenal axis suppression - [see Warnings and Precautions (5.1)] Symptoms of ...
-
7 DRUG INTERACTIONS7.1 CYP3A4 Inhibitors - Budesonide is a substrate for CYP3A4. Avoid use with CYP3A4 inhibitors. Concomitant oral administration of a strong CYP3A4 inhibitor (ketoconazole) caused an eight-fold ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited published studies report on the use of budesonide in pregnant women; however, the data are insufficient to inform a drug-associated risk for major birth ...
-
10 OVERDOSAGEReports of acute toxicity and/or death following overdosage of glucocorticoids are rare. Treatment consists of immediate gastric lavage or emesis followed by supportive and symptomatic therapy. If ...
-
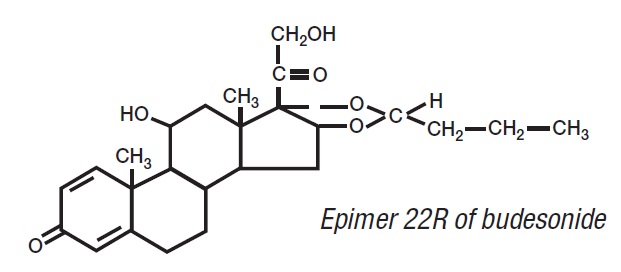
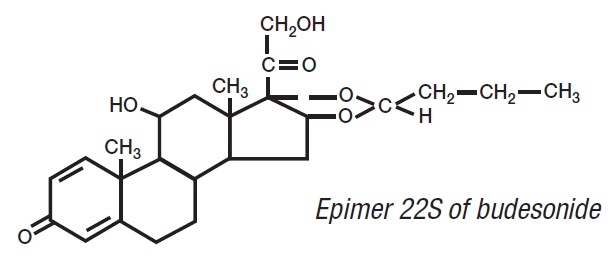
11 DESCRIPTIONBudesonide, the active ingredient of budesonide delayed-release capsules, is a synthetic corticosteroid. Budesonide is designated chemically as (RS)-11β, 16α ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Budesonide is an anti-inflammatory corticosteroid and has a high glucocorticoid effect and a weak mineralocorticoid effect, and the affinity of budesonide to ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies with budesonide were conducted in rats and mice. In a two-year study in Sprague-Dawley rats, budesonide caused ...
-
14 CLINICAL STUDIES14.1 Treatment of Mild to Moderate Active Crohn’s Disease - Adults - The efficacy of budesonide delayed-release capsules were evaluated in 994 patients with mild to moderate active Crohn’s ...
-
15 REFERENCES1. Best WR, Becktel JM, Singleton JW, Kern F: Development of a Crohn’s Disease Activity Index, National Cooperative Crohn’s Disease Study. Gastroenterology 1976; 70(3): 439-444.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGBudesonide 3 mg delayed-release capsules are hard gelatin capsules with an opaque light grey body and an opaque pink cap, coded with ENTOCORT EC 3 mg on the capsule and are supplied as ...
-
17 PATIENT COUNSELING INFORMATIONAdvise Patients to read the FDA-Approved patient labeling (Patient Information). Hypercorticism and Adrenal Axis Suppression - Advise patients that budesonide delayed-release capsules may cause ...
-
PATIENT INFORMATIONBudesonide (bue des' oh nide) delayed-release capsules - Read this Patient Information before you start taking budesonide delayed-release capsules and each time you get a refill. There may be new ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELNDC 51407-591-01 - Rx Only - Budesonide Delayed-Release Capsules 3mg - 100 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information