Label: NEYCHER BV AWAY- bacterial vaginosis symptom reliever suppository
- NDC Code(s): 84669-111-11
- Packager: NEYCHER INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
-
Warnings
For vaginal use only. Not for oral consumption.
Suppository may damage condoms and
diaphragms and cause them to fail.Do not use if ™ You have never had a yeast
infection diagnosed by a doctor. ™ If you are
allergic to any of the ingredients.Ask a doctor before use if you have ® Vaginal
itching and discomfort for the first time. = Lower
abdominal, back, or shoulder pain, fever, chills,
nausea, vomiting, or foul-smelling vaginal
discharge.When using this product = Do not use tampons,
douches, spermicides, or other vaginal products.
Stop use and ask a doctor if = Symptoms last
more than 7 days. ™ You get a rash or hives,
abdominal pain, fever, chills, nausea or vomiting.
If pregnant or breastfeeding, ask a health
professional before use. - KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- QUESTIONS
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NEYCHER BV AWAY
bacterial vaginosis symptom reliever suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84669-111 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THYMOL (UNII: 3J50XA376E) (THYMOL - UNII:3J50XA376E) THYMOL 3 [hp_C] CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 3 [hp_C] Inactive Ingredients Ingredient Name Strength ZINC OXIDE (UNII: SOI2LOH54Z) LACTIC ACID (UNII: 33X04XA5AT) ALOE VERA WHOLE (UNII: KIZ4X2EHYX) CHLORHEXIDINE (UNII: R4KO0DY52L) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) STEM BROMELAIN (UNII: ZLM4P8929R) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84669-111-11 10 in 1 BOX; Type 0: Not a Combination Product 11/04/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/04/2024 Labeler - NEYCHER INC (119362332) Establishment Name Address ID/FEI Business Operations KHARKIVSKA FARMATSEVTYCHNA FABRYKA TOV 681314127 manufacture(84669-111)


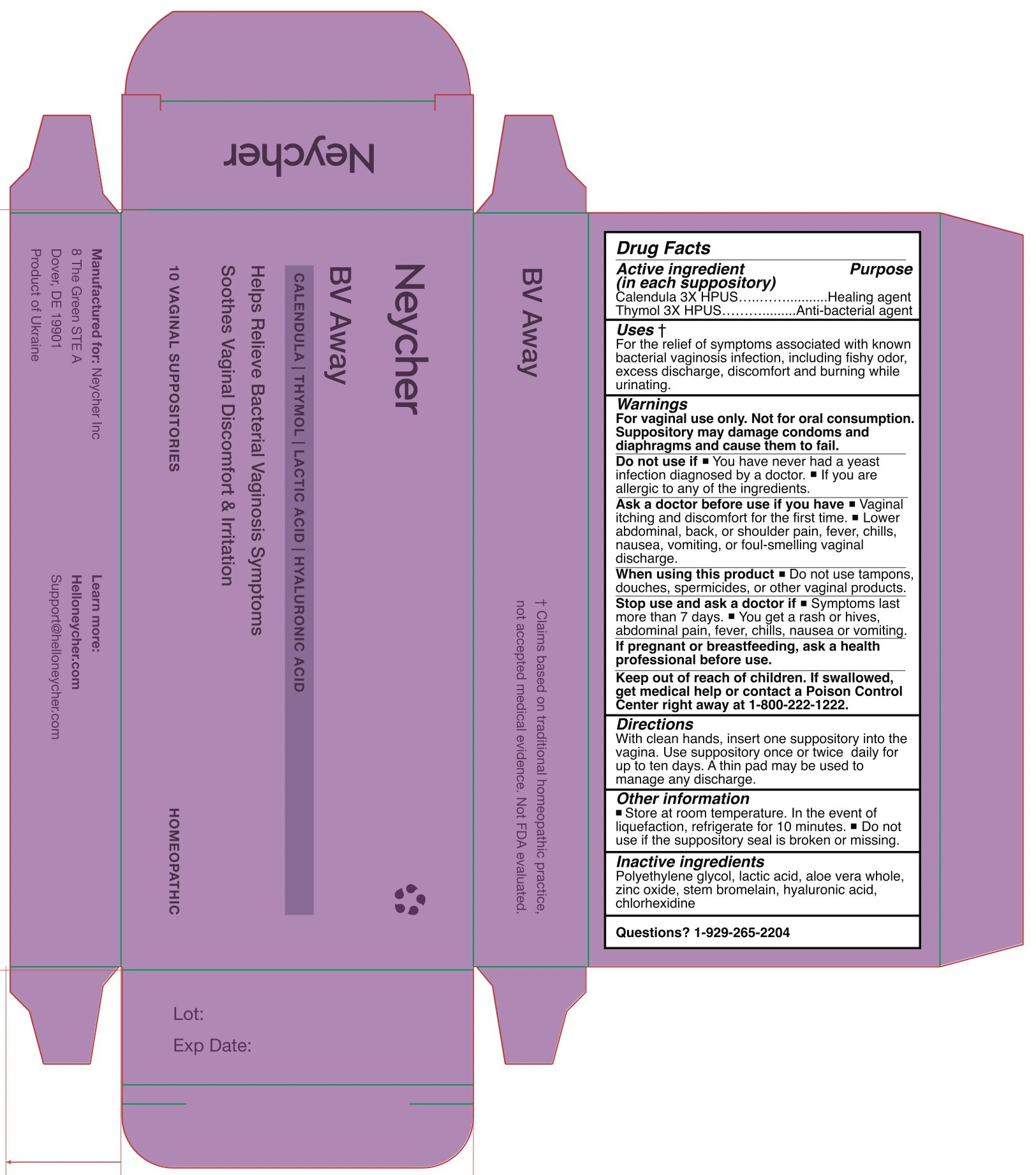 NDC: 84669-111-11
NDC: 84669-111-11