Label: AMINOCAPROIC ACID tablet
- NDC Code(s): 0904-7165-07
- Packager: Major Pharmaceuticals
- This is a repackaged label.
- Source NDC Code(s): 69238-1637
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
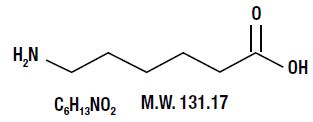
DESCRIPTIONAminocaproic acid is 6-aminohexanoic acid, which acts as an inhibitor of fibrinolysis. Its chemical structure is: Aminocaproic acid, USP is a fine, white to almost white, crystalline powder. It ...
-
CLINICAL PHARMACOLOGYThe fibrinolysis-inhibitory effects of aminocaproic acid appear to be exerted principally via inhibition of plasminogen activators and to a lesser degree through antiplasmin activity. In adults ...
-
INDICATIONS AND USAGEAminocaproic acid tablets are useful in enhancing hemostasis when fibrinolysis contributes to bleeding. In life-threatening situations, transfusion of appropriate blood products and other ...
-
CONTRAINDICATIONSAminocaproic acid should not be used when there is evidence of an active intravascular clotting process. When there is uncertainty as to whether the cause of bleeding is primary fibrinolysis or ...
-
WARNINGSIn patients with upper urinary tract bleeding, aminocaproic acid administration has been known to cause intrarenal obstruction in the form of glomerular capillary thrombosis or clots in the renal ...
-
PRECAUTIONSGeneral - Aminocaproic acid inhibits both the action of plasminogen activators and to a lesser degree, plasmin activity. The drug should NOT be administered without a definite diagnosis and/or ...
-
ADVERSE REACTIONSAminocaproic acid is generally well tolerated. The following adverse experiences have been reported: General: Edema, headache, malaise. Hypersensitivity Reactions: Allergic and anaphylactoid ...
-
OVERDOSAGEA few cases of acute overdosage with aminocaproic acid administered intravenously have been reported. The effects have ranged from no reaction to transient hypotension to severe acute renal ...
-
DOSAGE AND ADMINISTRATIONThe dosage regimen for administering aminocaproic acid tablets is as follows: For the treatment of acute bleeding syndromes due to elevated fibrinolytic activity, it is suggested that 10 ...
-
HOW SUPPLIEDAminocaproic Acid Tablets USP, 500 mg are supplied as round shape with notch, uncoated, biconvex, white to off-white tablets, debossed with identification marking “C” and “17” on either side of ...
-
REFERENCE1 Stefanini M, Dameshek W: The Hemorrhagic Disorders, Ed. 2, New York, Grune and Stratton; 1962: 510 to 514. Manufactured by: Amneal Pharmaceuticals Pvt. Ltd. Oral Solid Dosage ...
-
Package/Label Display Panel MAJOR® NDC 0904-7165-07 - Unit Dose - Aminocaproic Acid - Tablets, USP - 500 mg - 30 TABLETS (5 x 6) Rx only
-
INGREDIENTS AND APPEARANCEProduct Information