Label: GEMTESA- vibegron tablet, film coated
- NDC Code(s): 73336-075-07, 73336-075-30, 73336-075-90
- Packager: Sumitomo Pharma America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GEMTESA® safely and effectively. See full prescribing information for GEMTESA.
GEMTESA (vibegron) tablets, for oral use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
GEMTESA is a beta-3 adrenergic agonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency in adults. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 75 mg (3)
CONTRAINDICATIONS
Do not use if prior hypersensitivity reaction to vibegron or any components of the product. (4)
WARNINGS AND PRECAUTIONS
Urinary Retention: Monitor for urinary retention, especially in patients with bladder outlet obstruction and also in patients taking muscarinic antagonist medications for OAB, in whom the risk of urinary retention may be greater. If urinary retention develops, discontinue GEMTESA. (5.1)
ADVERSE REACTIONS
Most common adverse reactions (≥2%) reported with GEMTESA were headache, urinary tract infection, nasopharyngitis, diarrhea, nausea, and upper respiratory tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sumitomo Pharma America, Inc., at 1-833-876-8268 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Digoxin: Measure serum digoxin concentrations before initiating GEMTESA. Monitor serum digoxin concentrations to titrate digoxin dose to desired clinical effect. (7)
USE IN SPECIFIC POPULATIONS
Pediatric use: Safety and effectiveness in pediatric patients have not been established. (8.4)
End-stage Renal Disease with or without Hemodialysis: Not recommended. (8.6)
Severe Hepatic Impairment: Not recommended. (8.7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Urinary Retention
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of GEMTESA is one 75 mg tablet orally, once daily with or without food. Swallow GEMTESA tablets whole with a glass of water.
In adults, GEMTESA tablets also may be crushed, mixed with a tablespoon (approximately 15 mL) of applesauce and taken immediately with a glass of water [see Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
GEMTESA is contraindicated in patients with known hypersensitivity to vibegron or any components of the product [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Urinary Retention
Urinary retention has been reported in patients taking GEMTESA. The risk of urinary retention may be increased in patients with bladder outlet obstruction and also in patients taking muscarinic antagonist medications for the treatment of OAB. Monitor patients for signs and symptoms of urinary retention, particularly in patients with bladder outlet obstruction and patients taking muscarinic antagonist medications for the treatment of OAB. Discontinue GEMTESA in patients who develop urinary retention [see Adverse Reactions (6.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling:
- Urinary retention [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of GEMTESA was evaluated in a 12-week, double-blind, placebo- and active-controlled study (Study 3003) in patients with OAB [see Clinical Studies (14)]. A total of 545 patients received GEMTESA. The majority of the patients were Caucasian (78%) and female (85%) with a mean age of 60 years (range 18 to 93 years).
Adverse reactions that were reported in Study 3003 at an incidence greater than placebo and in ≥2% of patients treated with GEMTESA are listed in Table 1.
Table 1: Adverse Reactions, Exceeding Placebo Rate, Reported in ≥2% of Patients Treated with GEMTESA 75 mg for up to 12 Weeks in Study 3003 GEMTESA 75 mg
n (%)Placebo
n (%)Number of Patients 545 540 Headache 22 (4.0) 13 (2.4) Nasopharyngitis 15 (2.8) 9 (1.7) Diarrhea 12 (2.2) 6 (1.1) Nausea 12 (2.2) 6 (1.1) Upper respiratory tract infection 11 (2.0) 4 (0.7) Other adverse reactions reported in <2% of patients treated with GEMTESA included:
Gastrointestinal disorders: dry mouth, constipation
Investigations: residual urine volume increased
Renal and urinary disorders: urinary retention
Vascular disorders: hot flush
GEMTESA was also evaluated for long-term safety in an extension study (Study 3004) in 505 patients who completed the 12-week study (Study 3003). Of the 273 patients who received GEMTESA 75 mg once daily in the extension study, 181 patients were treated for a total of one year.
Adverse reactions reported in ≥2% of patients treated with GEMTESA 75 mg for up to 52 weeks in the long-term extension study, and not already listed above, were urinary tract infection (6.6%) and bronchitis (2.9%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of vibegron. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse events have been reported in association with vibegron use in worldwide postmarketing experience:
Urologic disorders: urinary retention
Skin and subcutaneous tissue disorders: pruritus, rash, drug eruption, eczema
Gastrointestinal disorders: constipation
-
7 DRUG INTERACTIONS
Concomitant use of GEMTESA increases digoxin maximal concentrations (Cmax) and systemic exposure as assessed by area under the concentration-time curve (AUC) [see Clinical Pharmacology (12.3)]. Serum digoxin concentrations should be monitored before initiating and during therapy with GEMTESA and used for titration of the digoxin dose to obtain the desired clinical effect. Continue monitoring digoxin concentrations upon discontinuation of GEMTESA and adjust digoxin dose as needed.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on GEMTESA use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
In animal studies, no effects on embryo-fetal development were observed following administration of vibegron during the period of organogenesis at exposures approximately 275-fold and 285-fold greater than clinical exposure at the recommended daily dose of GEMTESA, in rats and rabbits, respectively. Delayed fetal skeletal ossification was observed in rabbits at approximately 898-fold clinical exposure, in the presence of maternal toxicity. In rats treated with vibegron during pregnancy and lactation, no effects on offspring were observed at 89-fold clinical exposure. Developmental toxicity was observed in offspring at approximately 458-fold clinical exposure, in the presence of maternal toxicity. No effects on offspring were observed at 89-fold clinical exposure (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies carry some risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In an embryo-fetal developmental toxicity study, pregnant rats were treated with daily oral doses of 0, 30, 100, 300, or 1000 mg/kg/day vibegron during the period of organogenesis (Days 6 to 20 of gestation). These doses were associated with systemic exposures (AUC) 0-, 9-, 89-, 275-, and 1867-fold higher, respectively, than in humans treated with the recommended daily dose of GEMTESA. No embryo-fetal developmental toxicity was observed at doses up to 300 mg/kg/day. Treatment with the high dose of 1000 mg/kg/day was discontinued due to maternal toxicity.
In an embryo-fetal developmental toxicity study, pregnant rabbits were treated with daily oral doses of 0, 30, 100, or 300 mg/kg/day vibegron during the period of organogenesis (Days 7 to 20 of gestation). These doses were associated with systemic exposures (AUC) 0-, 86-, 285-, and 898-fold higher, respectively, than in humans treated with the recommended daily dose of GEMTESA. No embryo-fetal developmental toxicity was observed at doses of vibegron up to 100 mg/kg/day. Maternal toxicity (decreased food consumption), reduced fetal body weight, and an increased incidence of delayed skeletal ossification, were observed at 300 mg/kg/day.
In a pre- and post-natal developmental toxicity study, pregnant or lactating rats were treated with daily oral doses of 0, 30, 100, or 500 mg/kg/day vibegron from day 6 of gestation through day 20 of lactation. These doses were associated with estimated systemic exposures (AUC) 0-, 9-, 89-, and 458-fold higher, respectively, than in humans treated with the recommended daily dose of GEMTESA. No developmental toxicity was observed in F1 offspring at doses up to 100 mg/kg/day. Maternal toxicity was observed during lactation (decreased body weight gain) at doses ≥100 mg/kg/day and during gestation (decreased body weight gain and food consumption) at 500 mg/kg/day. Developmental toxicity was observed in F1 offspring (increased stillborn index, lethality, reduced viability and weaning indices, decreased body weight and body weight gains, low physical development differentiation indices, and effects on sensory function and reflexes) at 500 mg/kg/day.
8.2 Lactation
Risk Summary
There are no data on the presence of vibegron in human milk, the effects of the drug on the breastfed infant, or the effects on milk production. When a single oral dose of radiolabeled vibegron was administered to postnatal nursing rats, radioactivity was observed in milk (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for GEMTESA and any potential adverse effects on the breastfed infant from GEMTESA or from the underlying maternal condition.
Data
Animal Data
In a lactational transfer study, lactating rats were treated with a single oral dose of 10 mg/kg radiolabeled [3H] vibegron on postpartum day 10. Levels of radioactivity were determined in milk and plasma collected at 1, 4, 12, and 24 after dosing. The Cmax of total radioactivity in milk and plasma were observed at 9 and 2 hours after dosing, respectively, with a maximum milk-to-plasma concentration ratio of 2.2 observed at 12 hours after dosing. Vibegron elimination from milk showed a similar trend as that from plasma. The radioactivity concentration in milk at 24 hours after administration was approximately 25% of the Cmax.
8.4 Pediatric Use
The safety and effectiveness of GEMTESA in pediatric patients have not been established.
8.5 Geriatric Use
Of 526 patients who received GEMTESA in the clinical studies for OAB with symptoms of urge urinary incontinence, urgency, and urinary frequency, 242 (46%) were 65 years of age or older, and 75 (14%) were 75 years of age or older [see Clinical Studies (14)]. No overall differences in safety or effectiveness of GEMTESA have been observed between patients 65 years of age and older and younger adult patients.
8.6 Renal Impairment
No dosage adjustment for GEMTESA is recommended for patients with mild, moderate, or severe renal impairment (eGFR 15 to <90 mL/min/1.73 m2). GEMTESA has not been studied in patients with eGFR <15 mL/min/1.73 m2 (with or without hemodialysis) and is not recommended in these patients [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment for GEMTESA is recommended for patients with mild to moderate hepatic impairment (Child-Pugh A and B). GEMTESA has not been studied in patients with severe hepatic impairment (Child-Pugh C) and is not recommended in this patient population [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Vibegron is a selective beta-3 adrenergic agonist. The chemical name is (6S)-N-[4-[[(2S,5R)-5-[(R)-hydroxy(phenyl)methyl]pyrrolidin-2-yl]methyl]phenyl]-4-oxo-7,8-dihydro-6H-pyrrolo[1,2-a]pyrimidine-6-carboxamide having a molecular formula of C26H28N4O3 and a molecular weight of 444.538 g/mol. The structural formula of vibegron is:
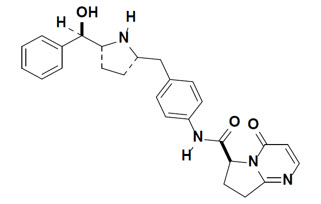
Vibegron is a crystalline, white to off-white to tan powder.
GEMTESA tablets, for oral administration contain 75 mg of vibegron and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, magnesium stearate, mannitol, and microcrystalline cellulose. The light green film coating contains FD&C Blue No. 2 - aluminum lake, hypromellose, iron oxide yellow, lactose monohydrate, titanium dioxide, and triacetin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Vibegron is a selective human beta-3 adrenergic receptor agonist. Activation of the beta-3 adrenergic receptor increases bladder capacity by relaxing the detrusor smooth muscle during bladder filling.
12.2 Pharmacodynamics
Vibegron's exposure-response relationship and the time course of pharmacodynamic response are not fully characterized.
Blood Pressure
In a 4-week, randomized, placebo-controlled, ambulatory blood pressure study in OAB patients (n=200), daily treatment with GEMTESA 75 mg was not associated with clinically significant changes in blood pressure. Subjects enrolled in this study had a mean age of 59 years and 75% were female. Thirty-five percent of subjects had pre-existing hypertension at baseline and 29% of all subjects were taking at least 1 concomitant antihypertensive medication.
12.3 Pharmacokinetics
Mean vibegron Cmax and AUC increased in a greater than dose-proportional manner up to 600 mg (8 times the approved recommended dosage). Steady state concentrations are achieved within 7 days of once daily dosing. The mean accumulation ratio (Rac) was 1.7 for Cmax and 2.4 for AUC0-24hr.
Absorption
Median vibegron Tmax is approximately 1 to 3 hours.
Oral administration of a 75 mg vibegron tablet crushed and mixed with 15 mL of applesauce resulted in no clinically relevant changes in vibegron pharmacokinetics when compared to administration of an intact 75 mg vibegron tablet.
Distribution
The mean apparent volume of distribution is 6304 liters. Human plasma protein binding of vibegron is approximately 50%. The average blood-to-plasma concentration ratio is 0.9.
Elimination
Vibegron has an effective half-life of 30.8 hours across all populations.
Specific Populations
No clinically significant differences in the pharmacokinetics of vibegron were observed based on age (18 to 93 years), sex, race/ethnicity (Japanese vs. non-Japanese), mild (eGFR 60 to <90 mL/min/1.73 m2), moderate (eGFR 30 to <60 mL/min/1.73 m2), and severe (eGFR 15 to <30 mL/min/1.73 m2) renal impairment, or moderate (Child-Pugh B) hepatic impairment. The effect of more severe renal impairment (eGFR <15 mL/min/1.73 m2) with or without hemodialysis or severe (Child-Pugh C) hepatic impairment on vibegron pharmacokinetics was not studied.
Drug Interaction Studies
Clinical Studies
Digoxin: Concomitant administration of vibegron increased digoxin Cmax and AUC by 21% and 11%, respectively.
Other Drugs: No clinically significant differences in vibegron pharmacokinetics were observed when used concomitantly with ketoconazole (P-gp and strong CYP3A4 inhibitor), diltiazem (P-gp and moderate CYP3A4 inhibitor), rifampin (strong CYP3A4 inducer), or tolterodine. No clinically significant differences in the pharmacokinetics of the following drugs were observed when used concomitantly with vibegron: tolterodine, tolterodine 5-hydroxy metabolite, metoprolol, combined oral contraceptive (ethinyl estradiol, levonorgestrel), or warfarin.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No carcinogenicity was observed in long-term studies conducted in mice and rats treated with daily oral doses of vibegron for approximately 2 years. In the mouse carcinogenicity study, CD-1 mice were treated with daily oral doses of vibegron up to 90 mg/kg/day in males and up to 150 mg/kg/day in females, corresponding to estimated systemic exposures (AUC) 21- and 55-fold higher, respectively, than in humans treated with the recommended daily dose of GEMTESA. In the rat carcinogenicity study, Sprague Dawley rats were treated with daily oral doses of vibegron up to 30 mg/kg/day in males and up to 180 mg/kg/day in females, corresponding to systemic exposures (AUC) 18- and 117-fold higher, respectively, than in humans treated with the recommended daily dose of GEMTESA.
Mutagenesis
Vibegron was not mutagenic in in vitro microbial reverse mutation assays, showed no evidence of genotoxic activity in an in vitro human peripheral blood lymphocyte chromosomal aberration assay, and did not increase the frequency of micronucleated polychromatic erythrocytes in an in vivo rat bone marrow micronucleus assay.
Impairment of Fertility
In fertility/general reproductive toxicity studies conducted in rats, females were treated with daily oral doses of 0, 30, 100, 300, or 1000 mg/kg/day vibegron and males were treated with daily oral doses of 0, 10, 30, or 300 mg/kg/day vibegron. No effects on fertility were observed in female or male rats at doses up to 300 mg/kg/day, associated with systemic exposure (AUC) at least 274-fold higher than in humans treated with the recommended daily dose of GEMTESA. General toxicity, decreased fecundity, and decreased fertility were observed in female rats at 1000 mg/kg/day, associated with estimated systemic exposure 1867-fold higher than in humans treated with the recommended daily dose of GEMTESA.
-
14 CLINICAL STUDIES
The efficacy of GEMTESA was evaluated in a 12-week, double-blind, randomized, placebo-controlled, and active-controlled trial (Study 3003, NCT03492281) in patients with OAB (urge urinary incontinence, urgency, and urinary frequency). Patients were randomized 5:5:4 to receive either GEMTESA 75 mg, placebo, or active control orally, once daily for 12 weeks. For study entry, patients had to have symptoms of OAB for at least 3 months with an average of 8 or more micturitions per day and at least 1 urge urinary incontinence (UUI) per day, or an average of 8 or more micturitions per day and an average of at least 3 urgency episodes per day. Urge urinary incontinence was defined as leakage of urine of any amount because the patient felt an urge or need to urinate immediately. The study population included OAB medication-naïve patients as well as patients who had received prior therapy with OAB medications.
The co-primary endpoints were change from baseline in average daily number of micturitions and average daily number of UUI episodes at week 12. Additional endpoints included change from baseline in average daily number of "need to urinate immediately" (urgency) episodes and average volume voided per micturition.
A total of 1,515 patients received at least one daily dose of placebo (n=540), GEMTESA 75 mg (n=545), or an active control treatment (n=430). The majority of patients were Caucasian (78%) and female (85%) with a mean age of 60 (range 18 to 93) years.
Table 2 shows changes from baseline at week 12 for average daily number of micturitions, average daily number of UUI episodes, average daily number of "need to urinate immediately" (urgency) episodes, and average volume voided per micturition.
Table 2: Mean Baseline and Change from Baseline at Week 12 for Micturition Frequency, Urge Urinary Incontinence Episodes, "Need to Urinate Immediately" (Urgency) Episodes, and Volume Voided per Micturition Parameter GEMTESA
75 mgPlacebo - *
- Least squares mean adjusted for treatment, baseline, sex, geographical region, study visit, and study visit by treatment interaction term.
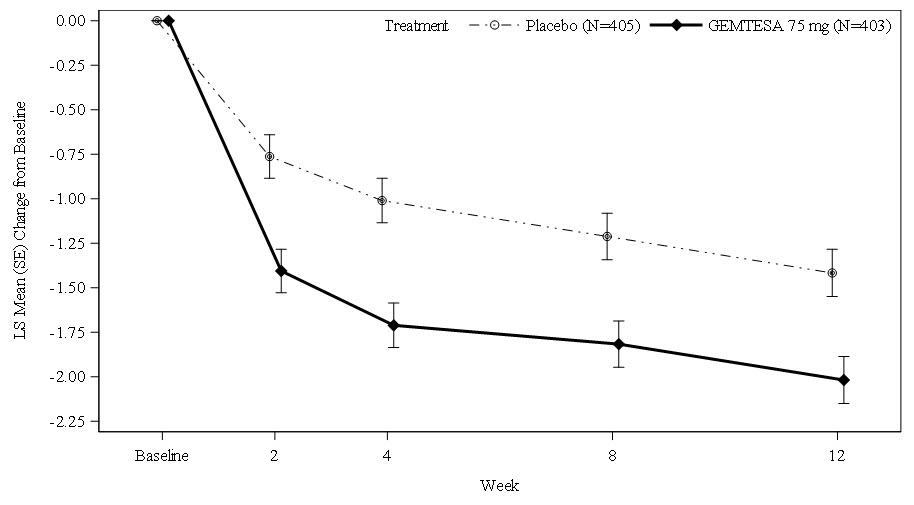
Average Daily Number of Micturitions Baseline mean (n) 11.3 (526) 11.8 (520) Change from Baseline* (n) -1.8 (492) -1.3 (475) Difference from Placebo -0.5 95% Confidence Interval -0.8, -0.2 p-value <0.001 Average Daily Number of UUI Episodes Baseline mean (n) 3.4 (403) 3.5 (405) Change from Baseline* (n) -2.0 (383) -1.4 (372) Difference from Placebo -0.6 95% Confidence Interval -0.9, -0.3 p-value <0.0001 Average Daily Number of "Need to Urinate Immediately" (Urgency) Episodes Baseline mean (n) 8.1 (526) 8.1 (520) Change from Baseline* (n) -2.7 (492) -2.0 (475) Difference from Placebo -0.7 95% Confidence Interval -1.1, -0.2 p-value 0.002 Average Volume Voided (mL) per Micturition Baseline mean (n) 155 (524) 148 (514) Change from Baseline* (n) 23 (490) 2 (478) Difference from Placebo 21 95% Confidence Interval 14, 28 p-value <0.0001 Figures 1 and 2 show the mean change from baseline over time in average daily number of micturitions and mean change from baseline over time in average daily number of UUI episodes, respectively.
Figure 1: Mean (SE) Change from Baseline in the Average Daily Number of Micturitions
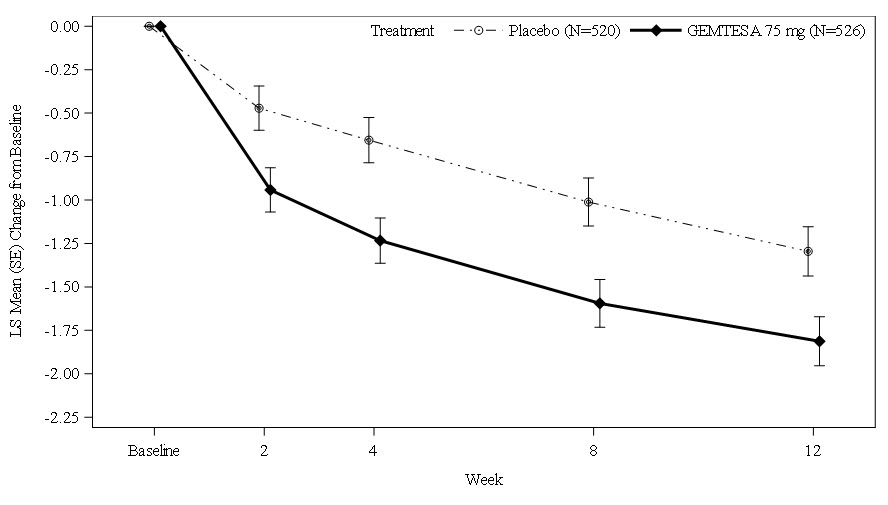
Figure 2: Mean (SE) Change from Baseline in the Average Daily Number of UUI Episodes in Patients with At Least 1 Average Daily UUI Episode at Baseline
-
16 HOW SUPPLIED/STORAGE AND HANDLING
GEMTESA 75 mg tablets are light green, oval, film-coated tablets, debossed with V75 on one side and no debossing on the other side.
GEMTESA is marketed in two packaging configurations:
Thirty (30) tablets in a 60 cc HDPE bottle with a child-resistant cap, NDC 73336-075-30
Ninety (90) tablets in a 60 cc HDPE bottle with a child-resistant cap, NDC 73336-075-90
Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Keep this and all medications out of sight and reach of children.
Dispose unused medication via a take-back option if available; otherwise follow FDA instructions for disposal in the household trash. See www.fda.gov/drugdisposal for more information.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Urinary Retention
Inform patients that GEMTESA has been associated with urinary retention. Inform patients that the risk of urinary retention may be increased in patients taking muscarinic antagonist medications for the treatment of OAB. Instruct patients to contact their healthcare provider if they experience symptoms consistent with urinary retention while taking GEMTESA [see Warnings and Precautions (5.1)].
Administration Instructions
Advise patients that GEMTESA tablets can be swallowed whole with a glass of water or may be crushed, mixed with a tablespoon of applesauce and taken immediately with a glass of water [see Dosage and Administration (2.1)].
-
SPL UNCLASSIFIED SECTION
Manufactured for and Distributed by:
Sumitomo Pharma America, Inc.
Marlborough, MA 01752 is a trademark of Sumitomo Pharma Co., Ltd., used under license.
is a trademark of Sumitomo Pharma Co., Ltd., used under license.
SUMITOMO PHARMA is a trademark of Sumitomo Pharma Co., Ltd., used under license.
SUMITOMO is a registered trademark of Sumitomo Chemical Co., Ltd., used under license. Sumitomo Pharma America, Inc. is a U.S. subsidiary of Sumitomo Pharma Co. Ltd.
All other trademarks are the property of their respective owners.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
GEMTESA [gem tes' ah]
(vibegron)
tablets, for oral useThis Patient Information has been approved by the U.S. Food and Drug Administration. Approved: 07/2023 What is GEMTESA?
GEMTESA is a prescription medicine for adults used to treat the following symptoms due to a condition called overactive bladder:- urge urinary incontinence: a strong need to urinate with leaking or wetting accidents
- urgency: the need to urinate right away
- frequency: urinating often
Do not take GEMTESA if you: - are allergic to vibegron or any of the ingredients in GEMTESA. See the end of this leaflet for a complete list of ingredients in GEMTESA.
Before you take GEMTESA, tell your doctor about all of your medical conditions, including if you: - have liver problems.
- have kidney problems.
- have trouble emptying your bladder or you have a weak urine stream.
- take medicines that contain digoxin.
- are pregnant or plan to become pregnant. It is not known if GEMTESA will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if GEMTESA passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take GEMTESA.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.How should I take GEMTESA? - Take GEMTESA exactly as your doctor tells you to take it.
- Take 1 GEMTESA tablet, by mouth, 1 time a day with or without food.
- Swallow GEMTESA tablets whole with a glass of water.
- You may also crush GEMTESA tablets, mix with 1 tablespoon (about 15 mL) of applesauce, and take right away with a glass of water.
What are the possible side effects of GEMTESA?
GEMTESA may cause serious side effects including:- inability to empty your bladder (urinary retention). GEMTESA may increase your chances of not being able to empty your bladder, especially if you have bladder outlet obstruction or take other medicines for treatment of overactive bladder. Tell your doctor right away if you are unable to empty your bladder.
- urinary tract infection
- headache
- nasal congestion, sore throat or runny nose
- diarrhea
- nausea
- upper respiratory tract infection
These are not all the possible side effects of GEMTESA. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store GEMTESA? - Store GEMTESA at room temperature between 68°F to 77°F (20°C to 25°C).
- Safely throw away medicine that is no longer needed in your household trash.
- You may also dispose of the unused medicine through a take-back option, if available. See www.fda.gov/drugdisposal for more information.
General information about the safe and effective use of GEMTESA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use GEMTESA for a condition for which it was not prescribed. Do not give GEMTESA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your doctor or pharmacist for information about GEMTESA that is written for health professionals.What are the ingredients in GEMTESA?
Active ingredient: vibegron
Inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, magnesium stearate, mannitol, and microcrystalline cellulose. The light green film coating contains FD&C Blue No. 2 - aluminum lake, hypromellose, iron oxide yellow, lactose monohydrate, titanium dioxide, and triacetin.Manufactured for and Distributed by:
Sumitomo Pharma America, Inc.
Marlborough, MA 01752
 is a trademark of Sumitomo Pharma Co., Ltd., used under license.
is a trademark of Sumitomo Pharma Co., Ltd., used under license.
SUMITOMO PHARMA is a trademark of Sumitomo Pharma Co., Ltd., used under license.
SUMITOMO is a registered trademark of Sumitomo Chemical Co., Ltd., used under license.
Sumitomo Pharma America, Inc. is a U.S. subsidiary of Sumitomo Pharma Co. Ltd.
All other trademarks are the property of their respective owners.
For more information, go to www.GEMTESA.com or call 1-833-876-8268.
- PRINCIPAL DISPLAY PANEL - 75 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
GEMTESA
vibegron tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73336-075 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength vibegron (UNII: M5TSE03W5U) (vibegron - UNII:M5TSE03W5U) vibegron 75 mg Inactive Ingredients Ingredient Name Strength mannitol (UNII: 3OWL53L36A) microcrystalline cellulose (UNII: OP1R32D61U) croscarmellose sodium (UNII: M28OL1HH48) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) magnesium stearate (UNII: 70097M6I30) FD&C BLUE NO. 2 ALUMINUM LAKE (UNII: 4AQJ3LG584) lactose monohydrate (UNII: EWQ57Q8I5X) hypromellose, unspecified (UNII: 3NXW29V3WO) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) titanium dioxide (UNII: 15FIX9V2JP) triacetin (UNII: XHX3C3X673) Product Characteristics Color GREEN Score no score Shape OVAL Size 9mm Flavor Imprint Code V75 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73336-075-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/29/2020 2 NDC:73336-075-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 12/29/2020 3 NDC:73336-075-07 1 in 1 CARTON 12/29/2020 3 7 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213006 12/29/2020 Labeler - Sumitomo Pharma America, Inc. (131661746)


