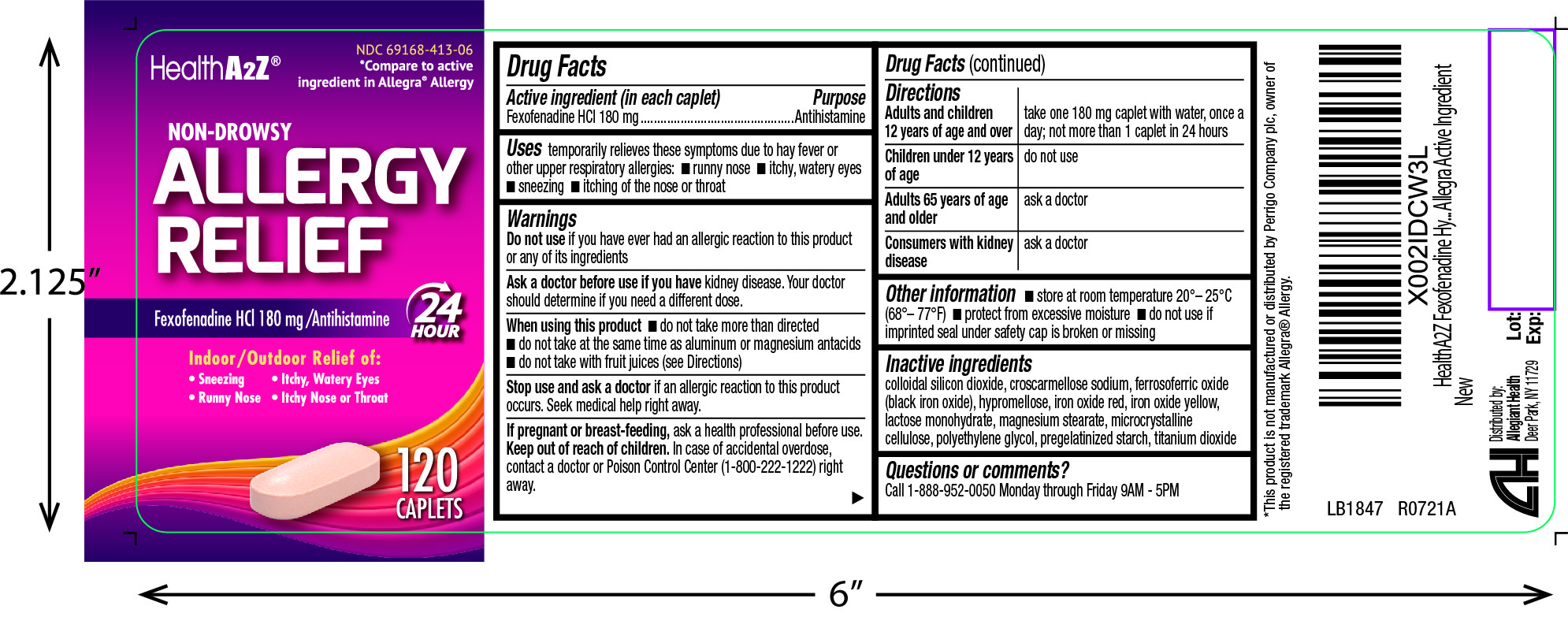
Label: ALLERGY RELIEF- fexofenadine hcl capsule, coated
- NDC Code(s): 69168-413-03, 69168-413-06, 69168-413-30
- Packager: Allegiant Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 23, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient(s)
- Purpose
- Use(s)
-
Warnings
Ask a doctor before use if
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- Directions
- Other information
- Storage
- Inactive ingredients
- Questions
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
fexofenadine hcl capsule, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69168-413 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score score with uneven pieces Shape CAPSULE Size 18mm Flavor Imprint Code G6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69168-413-03 3 in 1 CARTON; Type 0: Not a Combination Product 03/22/2021 2 NDC:69168-413-06 120 in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2021 3 NDC:69168-413-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211075 03/22/2021 Labeler - Allegiant Health (079501930)