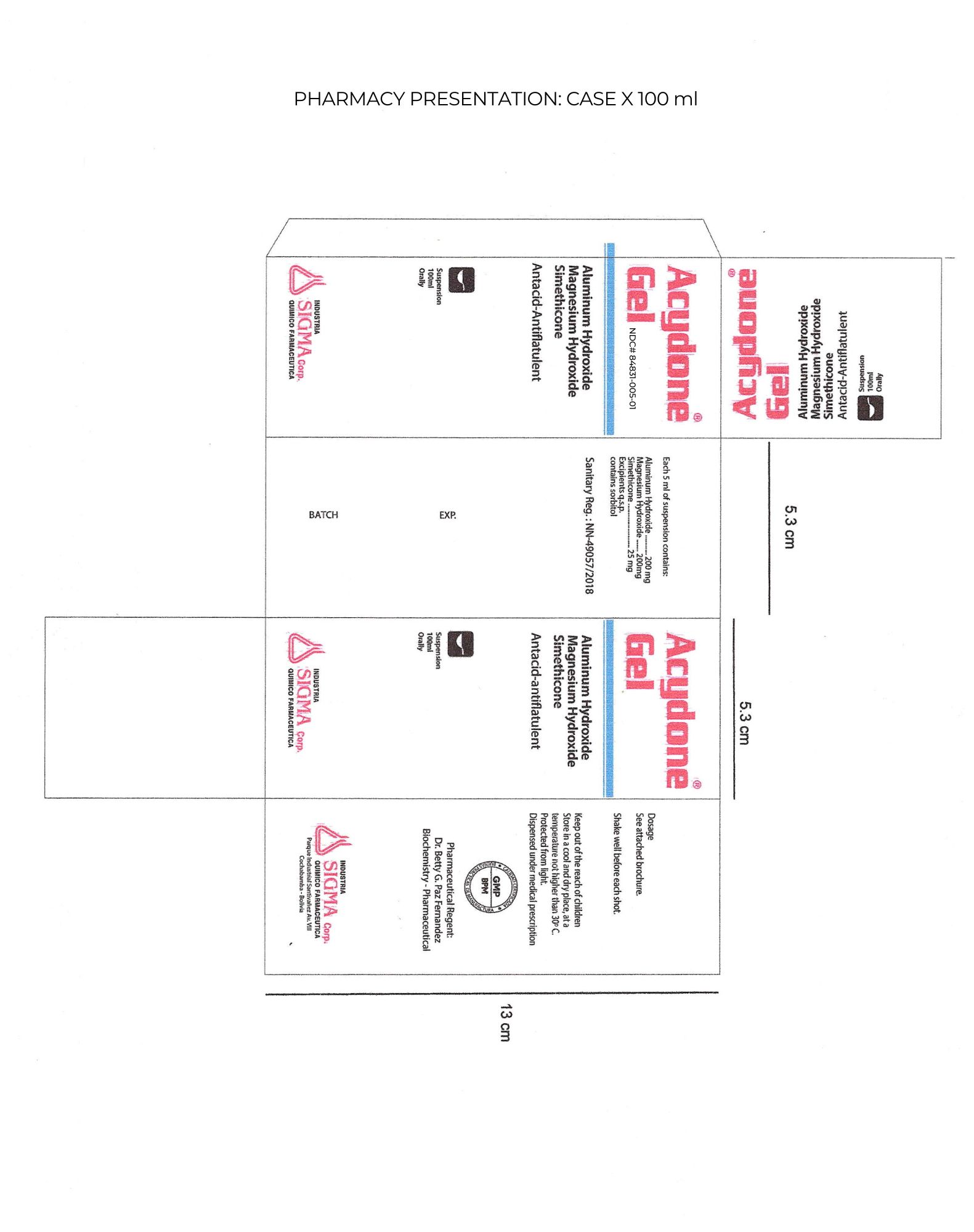
Label: ACYDONE- aluminum hydroxide, magnesium hydroxide, simethicone liquid
- NDC Code(s): 84831-005-01
- Packager: Sigma
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredient
Active ingredient (In each 5 ML Teaspoonful) Purpose
Aluminium Hydroxide 200 mg Antacid
Magnesium Hydroxide 200 mg Antacid
Simeticone 25 mg Anti-gasIndications and Usage
COMPOSITION Each 5 ml of Suspension contains:
Aluminum hydroxide………..…. 200 mg antacid
Magnesium Hydroxide ............. 200 mg antacid
Simethicone ………………….…. 25 mg antiflatulence
ACYDONE@ GEL is indicated in: Gastric hyperacidity of different origins, gastritis, esophagitis, flatulence, peptic ulcer, adjuvant in the treatment of gastroesophageal reflux, duodenitis, gastrointestinal disorders with excess gas. - Purpose
- Warning
- Directions
- Other Information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- Interior leaflet
-
INGREDIENTS AND APPEARANCE
ACYDONE
aluminum hydroxide, magnesium hydroxide, simethicone liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84831-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 25 mg in 5000 mg ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 200 mg in 5000 mg MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 200 mg in 5000 mg Inactive Ingredients Ingredient Name Strength PROPYLPARABEN (UNII: Z8IX2SC1OH) 1 mg in 5000 mg MINT (UNII: FV98Z8GITP) 0.25 mg in 5000 mg ALCOHOL (UNII: 3K9958V90M) 40 mg in 5000 mg WATER (UNII: 059QF0KO0R) 3558.3 mg in 5000 mg CHERRY (UNII: BUC5I9595W) 2.5 mg in 5000 mg GLYCERIN (UNII: PDC6A3C0OX) 500 mg in 5000 mg ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 0.45 mg in 5000 mg METHYLPARABEN (UNII: A2I8C7HI9T) 5 mg in 5000 mg XANTHAN GUM (UNII: TTV12P4NEE) 17.5 mg in 5000 mg SORBITOL (UNII: 506T60A25R) 400 mg in 5000 mg SODIUM SACCHARIN (UNII: SB8ZUX40TY) 50 mg in 5000 mg Product Characteristics Color white Score Shape Size Flavor CHERRY, MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84831-005-01 5000 mg in 1 BOTTLE; Type 0: Not a Combination Product 11/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/11/2024 Labeler - Sigma (815366311) Establishment Name Address ID/FEI Business Operations INDUSTRIA QUIMICO FARMACEUTICA SIGMA CORP. S.R.L. 815366311 manufacture(84831-005)