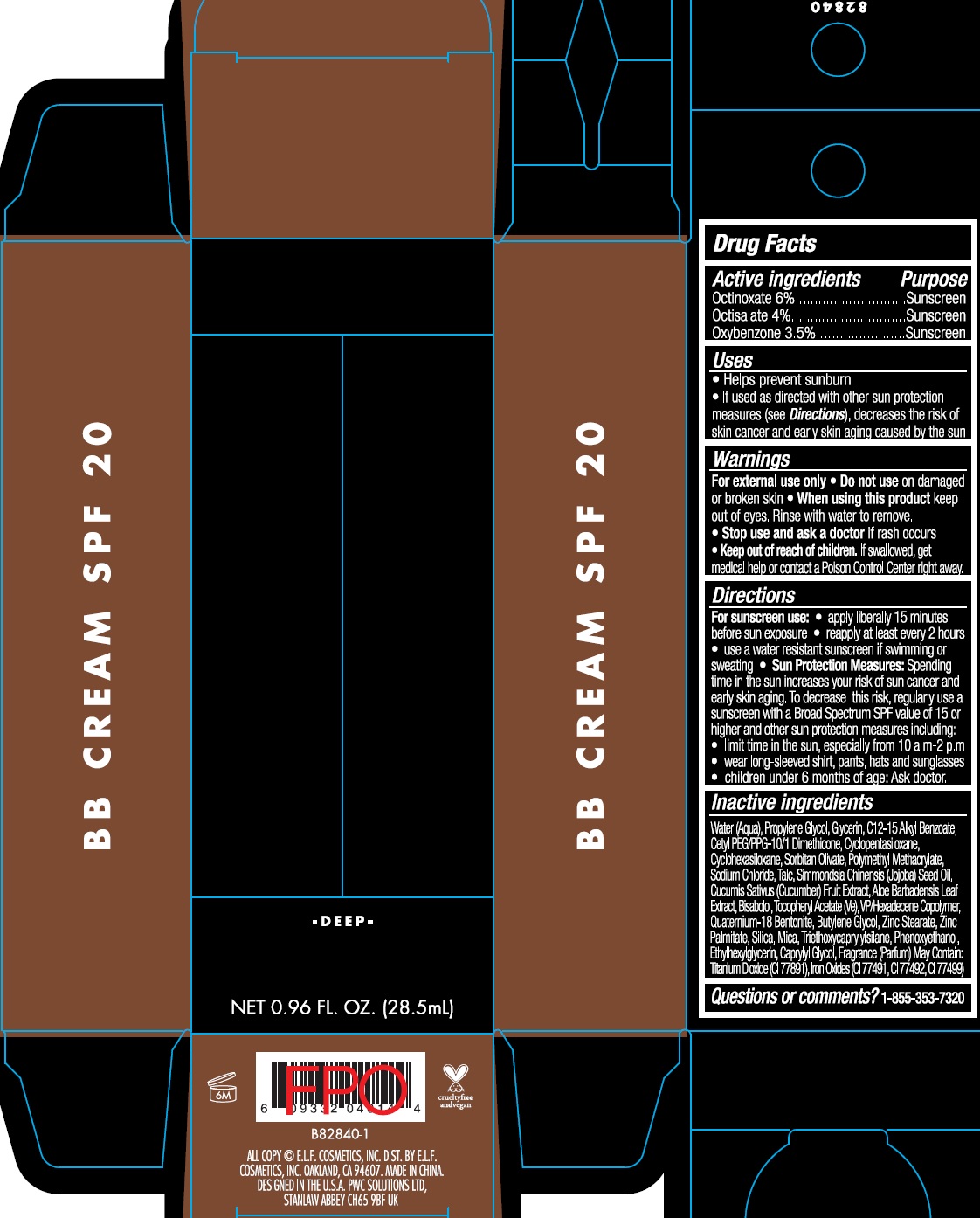
Label: BB SPF20- octinoxate, octisalate, oxybenzone cream
- NDC Code(s): 76354-450-28
- Packager: e.l.f. Cosmetics, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
- For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Spending time in the sun increases your risk of sun cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures:
- limit time in the sun, especially from 10 a.m.-2 p.m
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: Ask doctor.
-
Inactive ingredients
Water (Aqua), Propylene Glycol, Glycerin, C12-15 Alkyl Benzoate, Cetyl PEG/PPG-10/1 Dimethicone, Cyclopentasiloxane, Cyclohexasiloxane, Sorbitan Olivate, Polymethyl Methacrylate, Sodium Chloride, Talc, Simmondsia Chinensis (Jojoba) Seed Oil, Cucumis Sativus (Cucumber) Fruit Extract, Aloe Barbadensis Leaf Extract, Bisabolol, Tocopheryl Acetate (Ve), VP/Hexadecene Copolymer, Quaternium-18 Bentonite, Butylene Glycol, Zinc Stearate, Zinc Palmitate, Silica, Mica, Triethoxycaprylylsilane, Phenoxyethanol, Ethylhexylglycerin, Caprylyl Glycol, Fragrance (Parfum) May Contain: Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499)
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
BB SPF20
octinoxate, octisalate, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76354-450 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 40 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 35 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CYCLOMETHICONE 6 (UNII: XHK3U310BA) SORBITAN OLIVATE (UNII: MDL271E3GR) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) SODIUM CHLORIDE (UNII: 451W47IQ8X) TALC (UNII: 7SEV7J4R1U) JOJOBA OIL (UNII: 724GKU717M) CUCUMBER (UNII: YY7C30VXJT) ALOE VERA LEAF (UNII: ZY81Z83H0X) LEVOMENOL (UNII: 24WE03BX2T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) BENTOQUATAM (UNII: 7F465U79Q1) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ZINC STEARATE (UNII: H92E6QA4FV) ZINC PALMITATE (UNII: Q7407964JA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICA (UNII: V8A1AW0880) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76354-450-28 1 in 1 BOX 10/20/2019 1 28.5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/20/2019 12/12/2024 Labeler - e.l.f. Cosmetics, Inc (093902816)