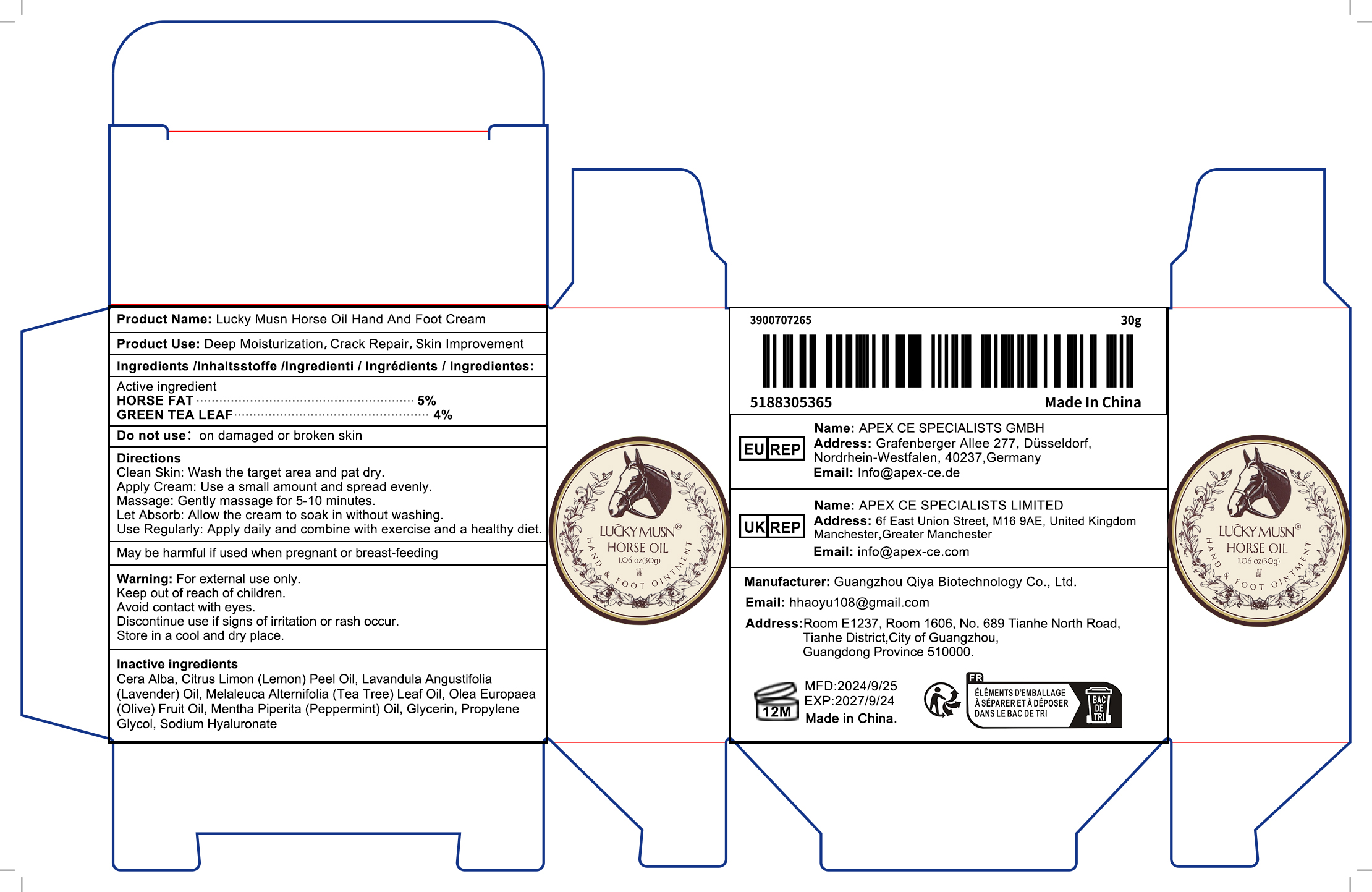
Label: LUCKY MUSN HORSE OIL HAND AND FOOT CREAM poultice
- NDC Code(s): 84793-003-01
- Packager: Guangzhou Qiya Biotechnology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose
- Uses
- Warnings
- Stop use and ask a doctor if
- Do not use
- When using this product
- Keep out of reach of children.
-
Dosage and Management
Recommended Amount: Approximately 10 grams per application.
Frequency of Use: Apply twice daily, morning and night.
Application Method: Clean the target area, apply evenly, and massage gently for 5-10 minutes.
Storage Conditions: Keep in a cool, dry place away from direct sunlight.
Allergy Test: Conduct a patch test on a small area before full use.
Warning: Discontinue use if any adverse reactions occur and consult a professional.
- inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LUCKY MUSN HORSE OIL HAND AND FOOT CREAM
lucky musn horse oil hand and foot cream poulticeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84793-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HORSE FAT (UNII: 9C68YEU7DP) (HORSE FAT - UNII:9C68YEU7DP) HORSE FAT 2 g in 2 g GREEN TEA LEAF (UNII: W2ZU1RY8B0) (GREEN TEA LEAF - UNII:W2ZU1RY8B0) GREEN TEA LEAF 2 g in 2 g Inactive Ingredients Ingredient Name Strength SODIUM HYALURONATE (UNII: YSE9PPT4TH) 2 g in 2 g LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL (UNII: ZBP1YXW0H8) 2 g in 2 g MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL (UNII: VIF565UC2G) 2 g in 2 g Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84793-003-01 1 g in 1 BOTTLE; Type 0: Not a Combination Product 10/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 10/30/2024 Labeler - Guangzhou Qiya Biotechnology Co., Ltd (412478719) Establishment Name Address ID/FEI Business Operations Guangzhou Qiya Biotechnology Co., Ltd 412478719 manufacture(84793-003)