Label: SULFASALAZINE tablet, delayed release
- NDC Code(s): 59762-0104-1, 59762-0104-2, 59762-0104-5, 59762-0104-6
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated May 29, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONEnteric-coated Tablets
-
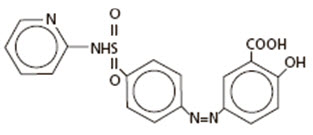
DESCRIPTIONSulfasalazine delayed release tablets contain sulfasalazine, formulated in a delayed release tablet (enteric-coated), 500 mg, for oral administration. Sulfasalazine delayed release tablets are ...
-
CLINICAL PHARMACOLOGYPharmacodynamics - The mode of action of sulfasalazine (SSZ) or its metabolites, 5-aminosalicylic acid (5-ASA) and sulfapyridine (SP), may be related to the anti-inflammatory and/or ...
-
INDICATIONS AND USAGESulfasalazine delayed release tablets are indicated: a) in the treatment of mild to moderate ulcerative colitis, and as adjunctive therapy in severe ulcerative colitis; b) for the prolongation of ...
-
CONTRAINDICATIONSSulfasalazine delayed release tablets are contraindicated in: Hypersensitivity to sulfasalazine, its metabolites, sulfonamides or salicylates, Patients with intestinal or urinary ...
-
WARNINGSHepatic, Renal, and Hematologic Toxicity or Other Conditions - Only after critical appraisal should sulfasalazine delayed release tablets be given to patients with hepatic or renal damage or ...
-
PRECAUTIONSGeneral: Sulfasalazine delayed release tablets should be given with caution to patients with severe allergy or bronchial asthma. Adequate fluid intake must be maintained in order to prevent ...
-
ADVERSE REACTIONSThe most common adverse reactions associated with sulfasalazine in ulcerative colitis are anorexia, headache, nausea, vomiting, gastric distress, and apparently reversible oligospermia. These ...
-
DRUG ABUSE AND DEPENDENCENone reported.
-
OVERDOSAGEThere is evidence that the incidence and severity of toxicity following overdosage is directly related to the total serum sulfapyridine concentration. Symptoms of overdosage may include nausea ...
-
DOSAGE AND ADMINISTRATIONThe dosage of sulfasalazine delayed release tablets should be adjusted to each individual's response and tolerance. Patients should be instructed to take sulfasalazine delayed release tablets in ...
-
HOW SUPPLIEDSulfasalazine delayed release tablets, 500 mg, are elliptical, gold-colored, film enteric-coated tablets, monogrammed "104" on one side. They are available in the following package ...
-
REFERENCES1. Van Rossum MAJ, et al. Sulfasalazine in the treatment of juvenile chronic arthritis: a randomized, double-blind, placebo-controlled, multicenter study. Arth Rheum 1998; 41:808–816. 2. Mogadam ...
-
SPL UNCLASSIFIED SECTIONLAB-0238-18.0 - Revised: 02/2025
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle LabelNDC 59762-0104-1 - 100 Enteric-coated Tablets - GREENSTONE® BRAND - sulfasalazine delayed - release tablets, USP - 500 mg - Rx only
-
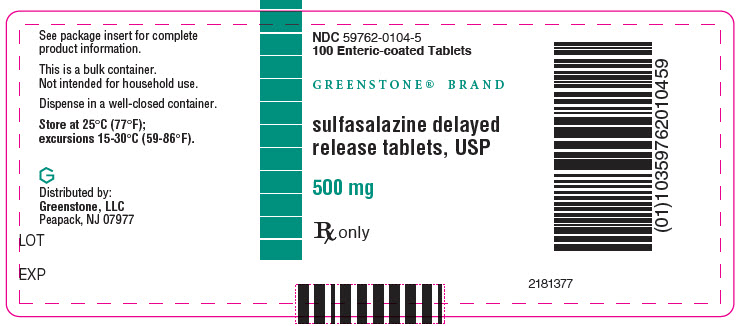
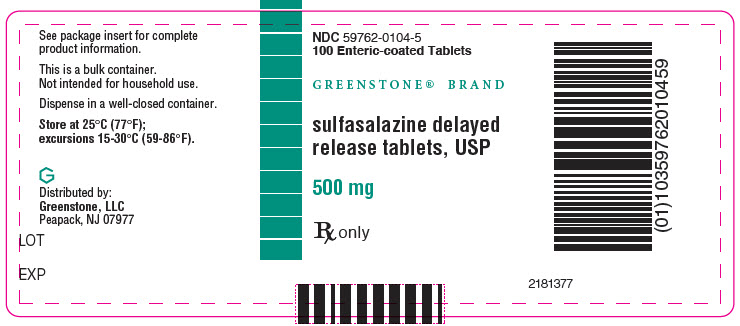
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label - NDC 59762-0104-5NDC 59762-0104-5 - 100 Enteric-coated Tablets - GREENSTONE® BRAND - sulfasalazine delayed - release tablets, USP - 500 mg - Rx only
-
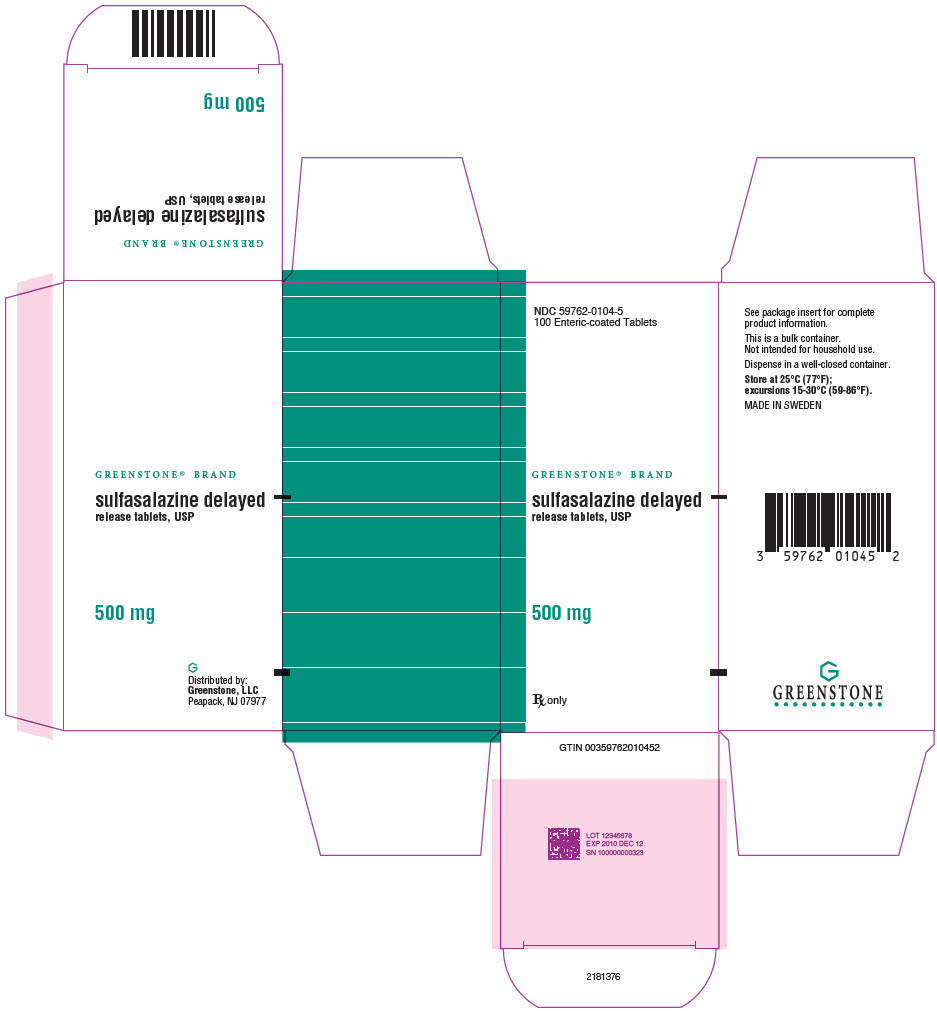
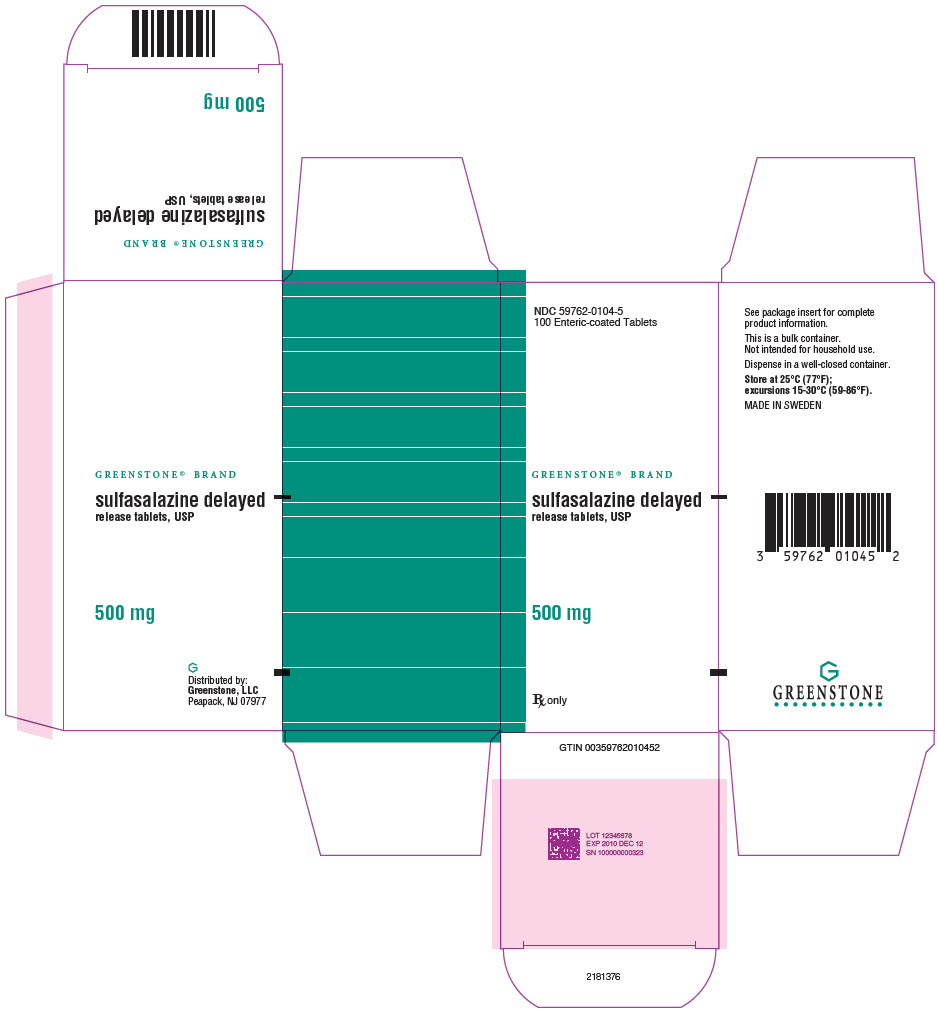
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Carton - NDC 59762-0104-5NDC 59762-0104-5 - 100 Enteric-coated Tablets - GREENSTONE® BRAND - sulfasalazine delayed - release tablets, USP - 500 mg - Rx only
-
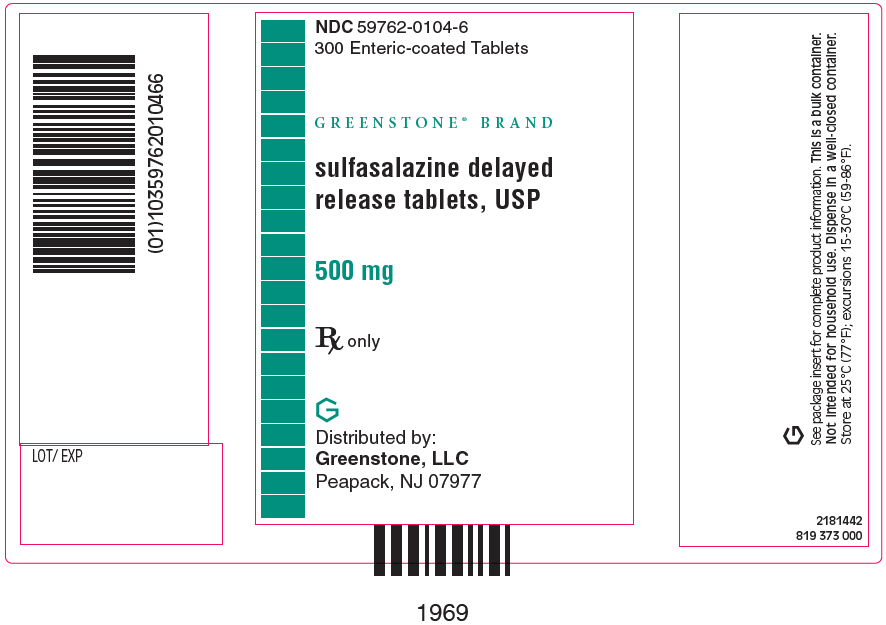
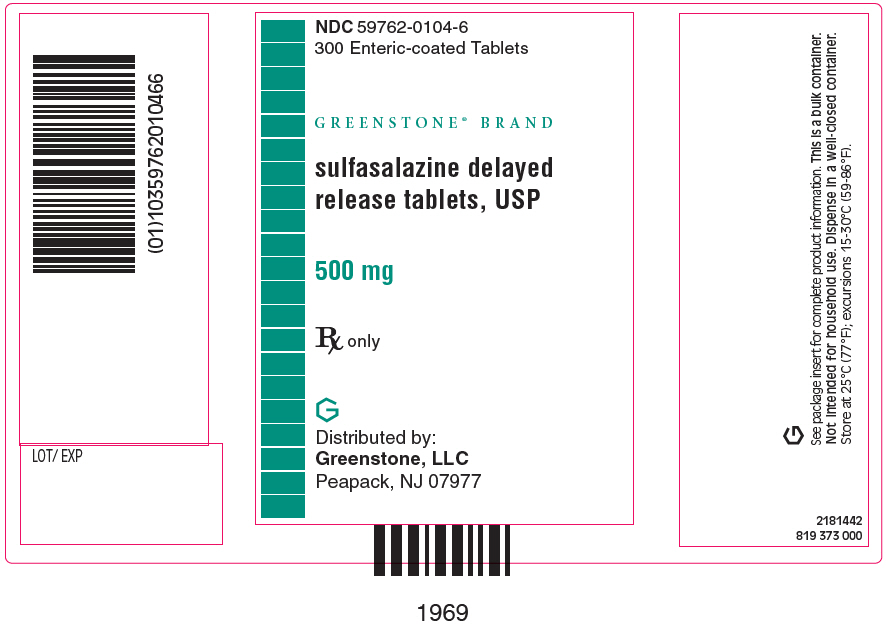
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label - NDC 59762-0104-6NDC 59762-0104-6 - 300 Enteric-coated Tablets - GREENSTONE® BRAND - sulfasalazine delayed - release tablets, USP - 500 mg - Rx only - Distributed by: Greenstone, LLC - Morgantown, WV26505 U.S.A.
-
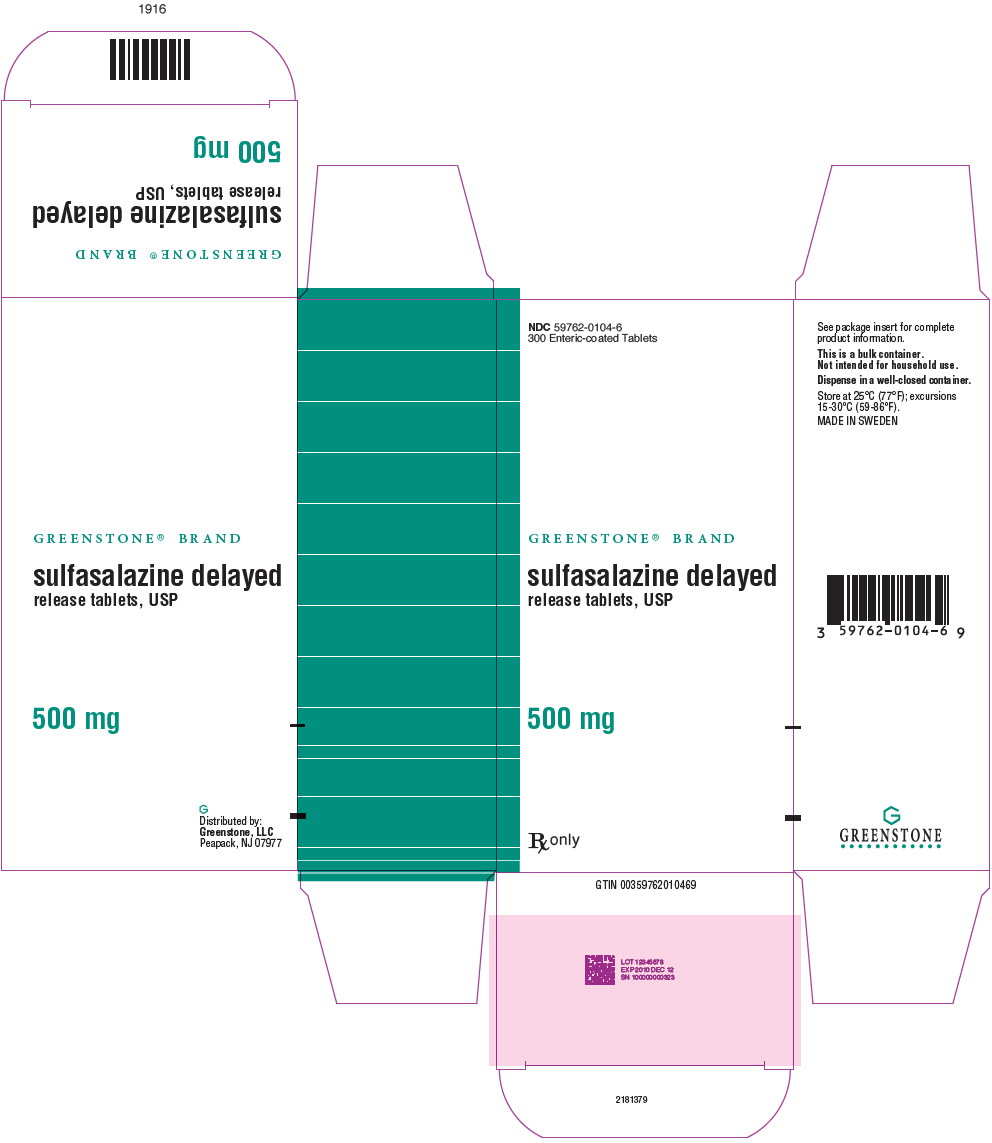
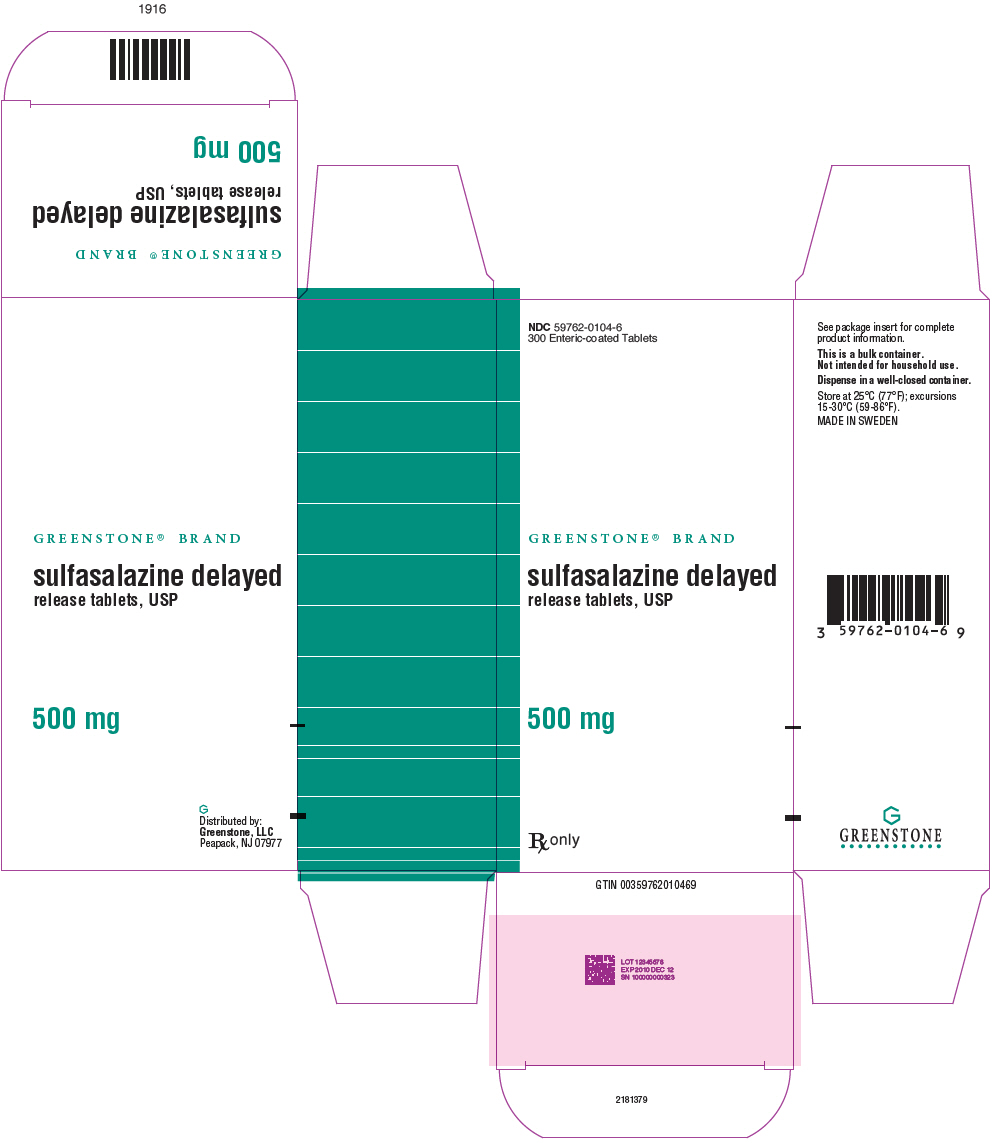
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Carton - NDC 59762-0104-6NDC 59762-0104-6 - 300 Enteric-coated Tablets - GREENSTONE® BRAND - sulfasalazine delayed - release tablets, USP - 500 mg - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information