Label: ZINGO- lidocaine hydrochloride monohydrate powder
- NDC Code(s): 61388-123-12, 61388-123-26, 61388-123-48
- Packager: Powder Pharmaceutical Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZINGO safely and effectively. See full prescribing information for ZINGO. ZINGO (lidocaine hydrochloride monohydrate) powder ...
-
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE - 2 DOSAGE AND ADMINISTRATION - 2.1 Instructions for Use - 3 DOSAGE FORMS AND STRENGTHS - 4 CONTRAINDICATIONS - 5 WARNINGS AND PRECAUTIONS - 5.1 Methemoglobinemia - 6 ...
-
1 INDICATIONS AND USAGE
ZINGO is indicated for use on intact skin to provide topical local analgesia prior to venipuncture or peripheral intravenous cannulation, in children 3–18 years of age. ZINGO is indicated for use ...
-
2 DOSAGE AND ADMINISTRATION
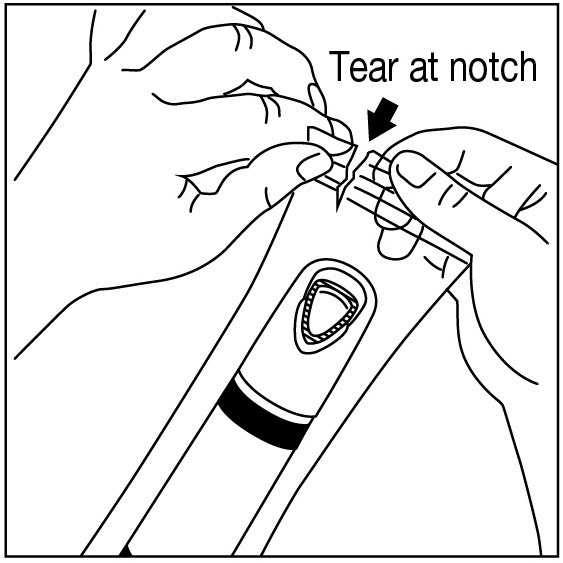
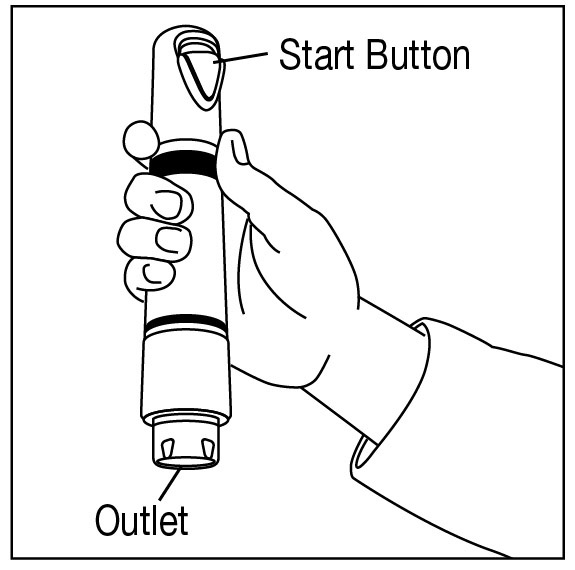
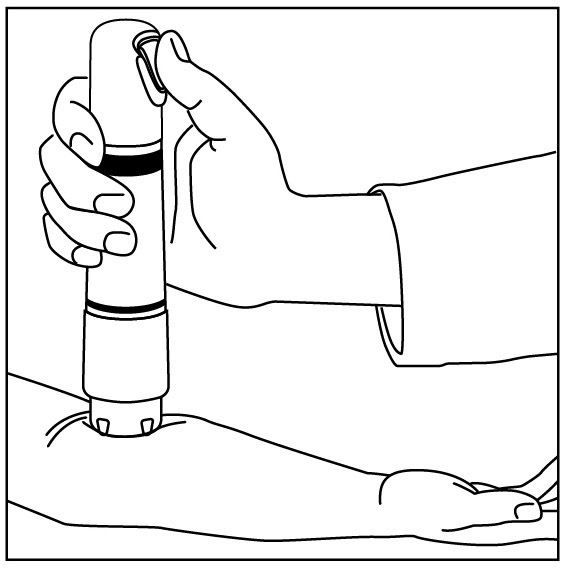
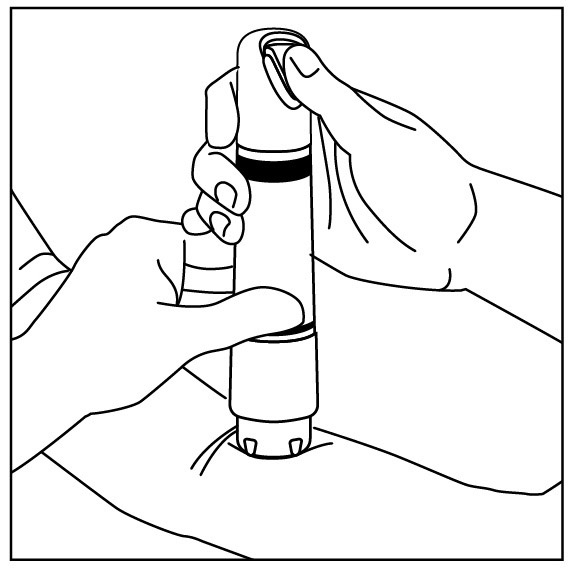
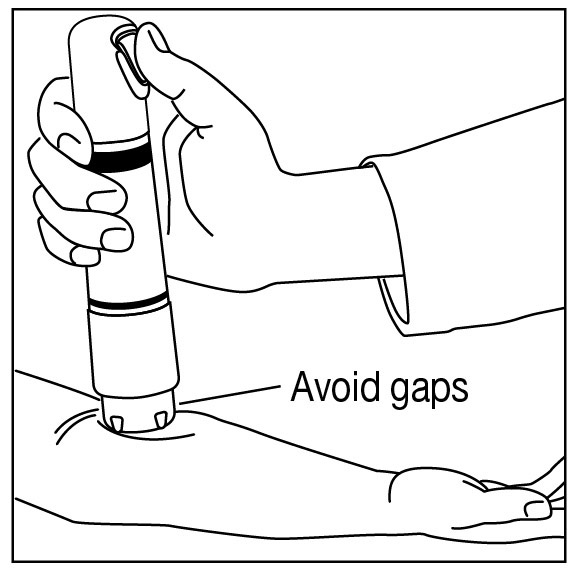
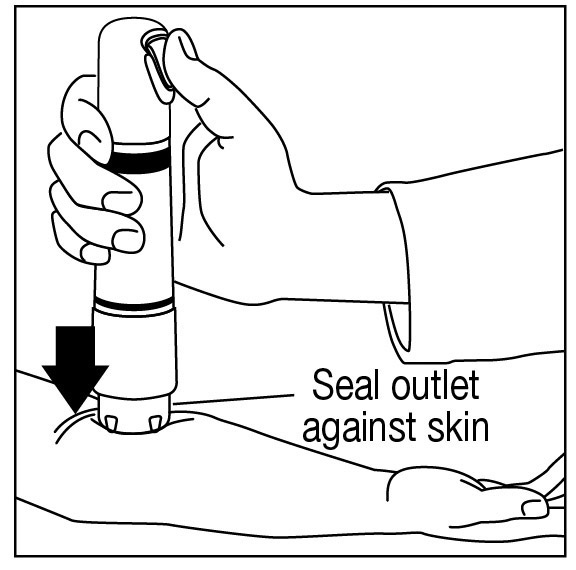
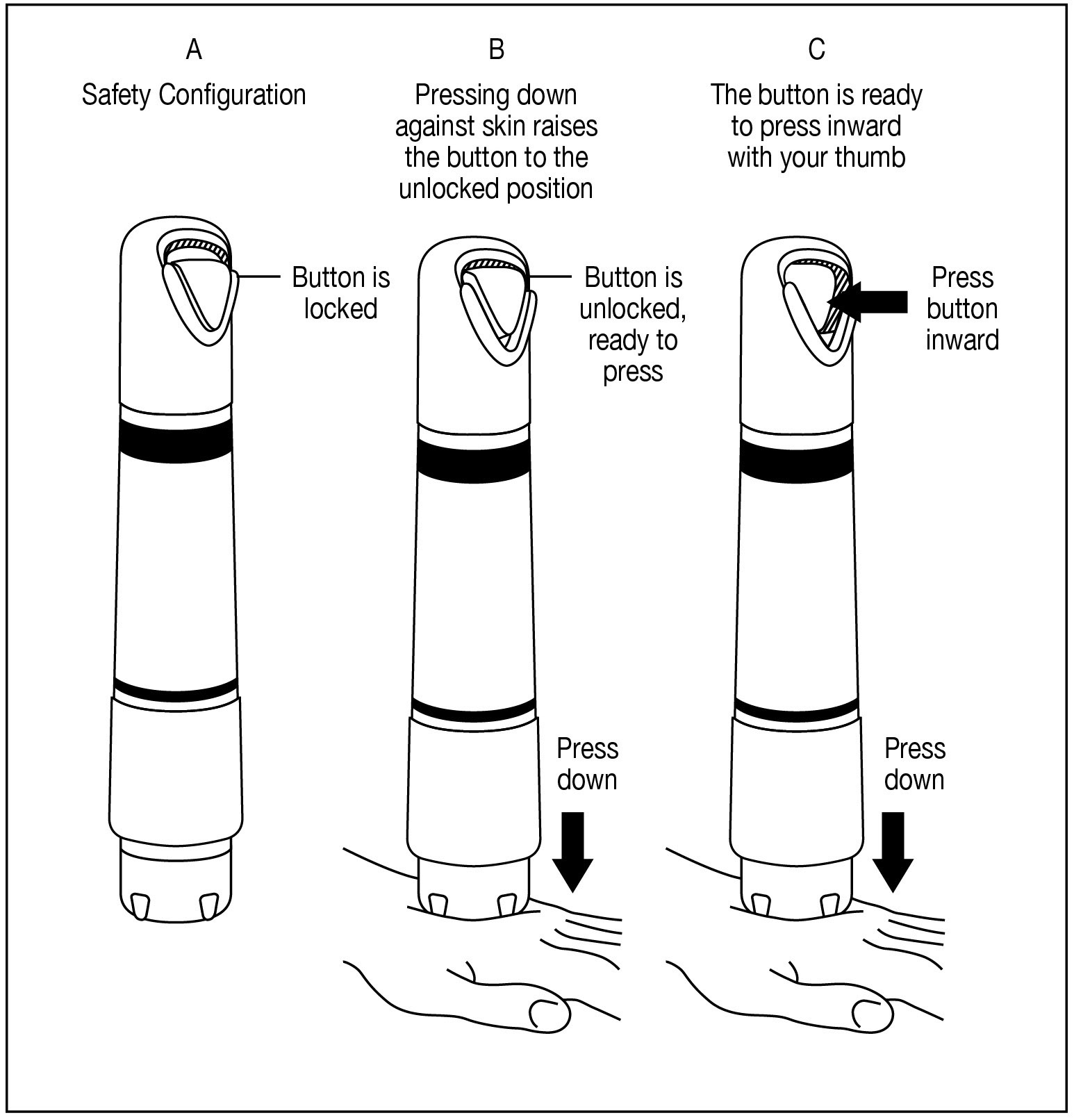
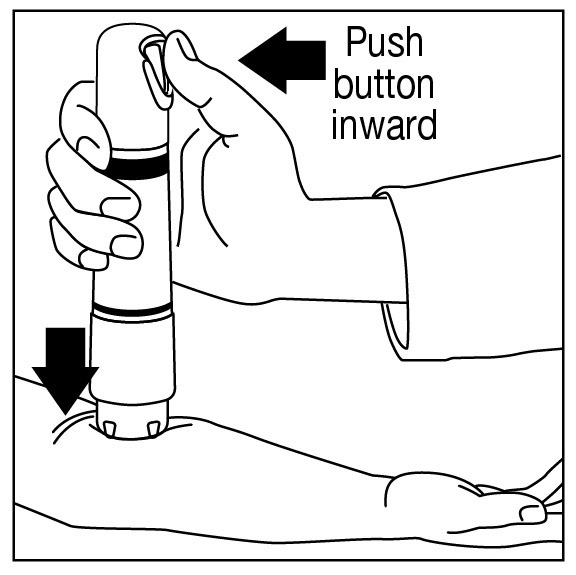
Apply one ZINGO (0.5 mg lidocaine hydrochloride monohydrate) to the site planned for venipuncture or intravenous cannulation, one to three minutes prior to needle insertion. Perform the procedure ...
-
3 DOSAGE FORMS AND STRENGTHS
ZINGO (lidocaine hydrochloride monohydrate) powder intradermal injection system contains 0.5 mg of sterile lidocaine hydrochloride monohydrate.
-
4 CONTRAINDICATIONS
ZINGO is contraindicated in patients with a known history of sensitivity to local anesthetics of the amide type.
-
5 WARNINGS AND PRECAUTIONS
Do not use around the eyes. Do not use ZINGO on body orifices, mucous membranes, or on areas with a compromised skin barrier. Only use ZINGO on skin locations where an adequate seal can be ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - ZINGO was not formally evaluated for effects on reproduction. Significant systemic exposure to lidocaine is not expected under recommended conditions of use of ZINGO as ...
-
10 OVERDOSAGE
In adults following a single administration of ZINGO the plasma levels of lidocaine were below the limit of detection (5 ng/mL). Signs of central nervous system (CNS) toxicity may start at plasma ...
-
11 DESCRIPTION
ZINGO - ® (lidocaine hydrochloride monohydrate) powder intradermal injection system contains 0.5 mg of sterile lidocaine hydrochloride monohydrate. The chemical name is ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - ZINGO delivers lidocaine hydrochloride monohydrate into the dermis. Lidocaine is an amide-type local anesthetic agent that blocks sodium ion channels required for ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals have not been performed to evaluate the carcinogenic potential of ...
-
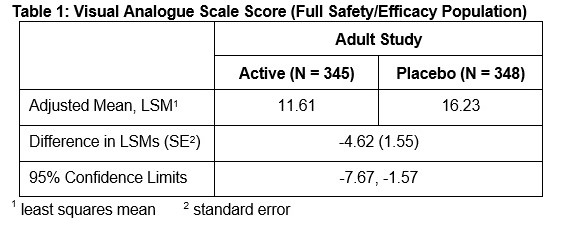
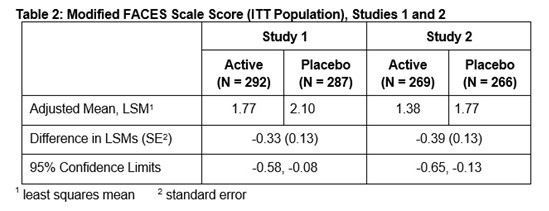
14 CLINICAL STUDIES
Efficacy in Adults - The efficacy of ZINGO in adults was evaluated in a randomized, double-blind, parallel-arm, sham-placebo controlled trial in which adult patients who required a venipuncture ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC 70645-123 ZINGO - ® (lidocaine hydrochloride monohydrate) powder intradermal injection system contains 0.5 mg of sterile lidocaine hydrochloride monohydrate. ZINGO - ® is a single-dose device ...
-
17 PATIENT COUNSELING INFORMATION
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if ...
-
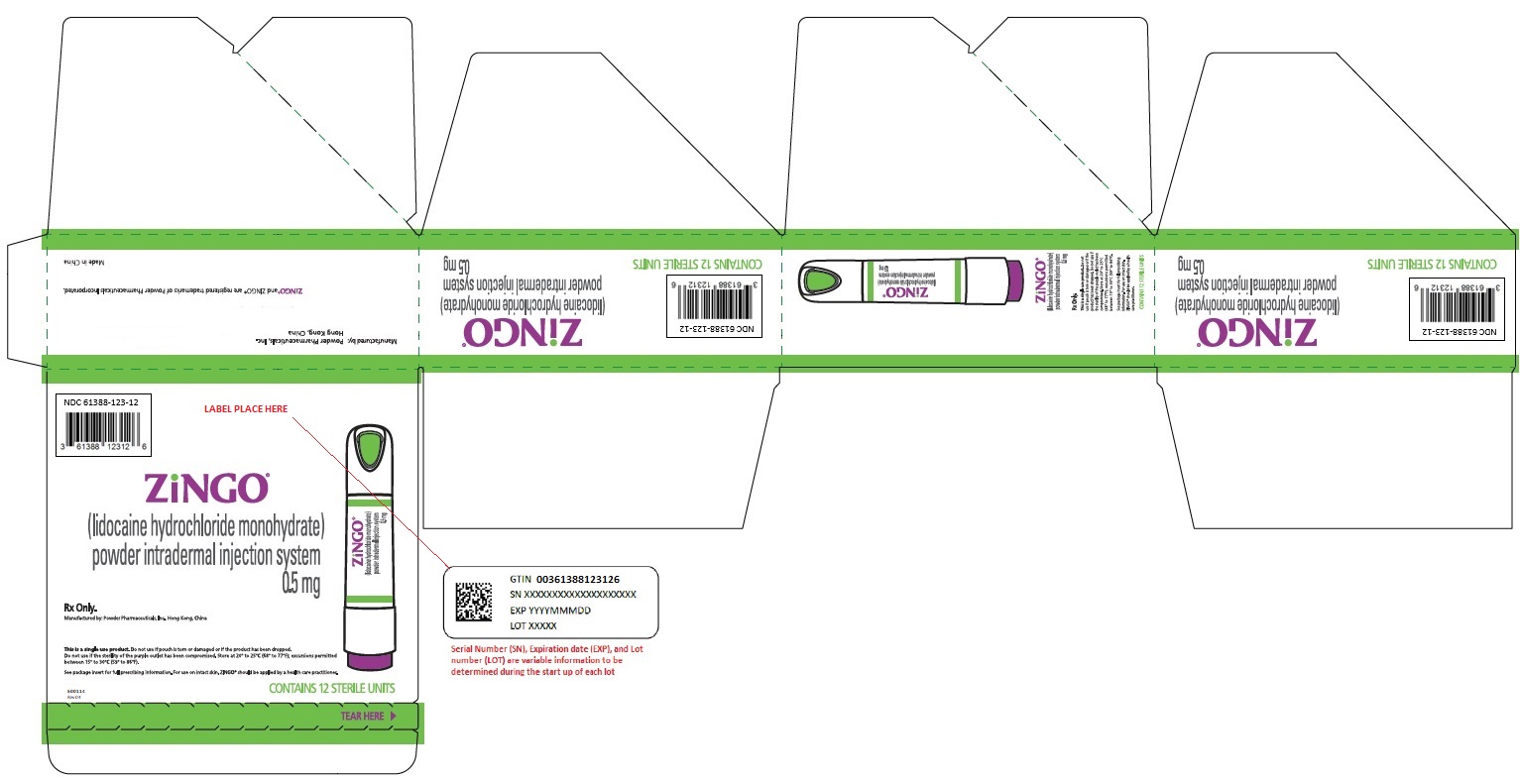
OUTER PACKAGING and PRINCIPAL DISPLAY PANEL
Device Label - NDC 61388-123-26 - ZINGO® (lidocaine hydrochloride monohydrate) powder intradermal injection system 0.5 mg - contains 1 sterile unit - Rx Only - Carton Label ...
-
INGREDIENTS AND APPEARANCEProduct Information