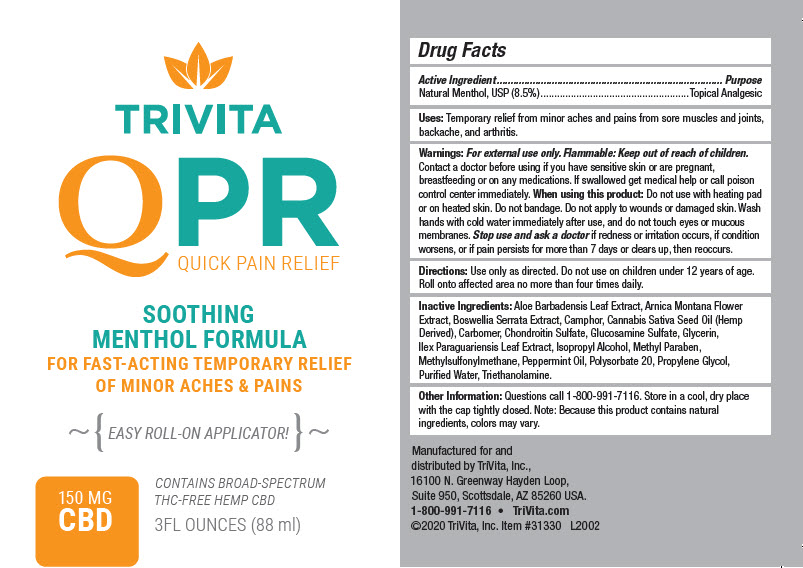
Label: TRIVITA QPR- menthol, unspecified form gel
- NDC Code(s): 76571-313-30
- Packager: TriVita, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only.
Flammable: Keep out of reach of children.
Contact a doctor before using if you have sensitive skin or are pregnant, breastfeeding or on any medications. If swallowed get medical help or call poison control center immediately.
- Directions
-
Inactive Ingredients
Aloe Barbadensis Leaf Extract, Arnica Montana Flower Extract, Boswellia Serrata Extract, Camphor, Cannabis Sativa Seed Oil (Hemp Derived), Carbomer, Chondroitin Sulfate, Glucosamine Sulfate, Glycerin, Ilex Paraguariensis Leaf Extract, Isopropyl Alcohol, Methyl Paraben, Methylsulfonylmethane, Peppermint Oil, Polysorbate 20, Propylene Glycol, Purified Water, Triethanolamine.
- Other Information
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 88 ml Bottle Label
-
INGREDIENTS AND APPEARANCE
TRIVITA QPR
menthol, unspecified form gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76571-313 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 8.5 g in 100 mL Inactive Ingredients Ingredient Name Strength Aloe Vera Leaf (UNII: ZY81Z83H0X) Arnica Montana Flower (UNII: OZ0E5Y15PZ) Indian Frankincense (UNII: 4PW41QCO2M) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) Carbomer Homopolymer, Unspecified Type (UNII: 0A5MM307FC) Glucosamine Sulfate (UNII: 1FW7WLR731) Glycerin (UNII: PDC6A3C0OX) Ilex Paraguariensis Leaf (UNII: 1Q953B4O4F) Isopropyl Alcohol (UNII: ND2M416302) MethylParaben (UNII: A2I8C7HI9T) Dimethyl Sulfone (UNII: 9H4PO4Z4FT) Peppermint Oil (UNII: AV092KU4JH) Polysorbate 20 (UNII: 7T1F30V5YH) Propylene Glycol (UNII: 6DC9Q167V3) Water (UNII: 059QF0KO0R) Trolamine (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76571-313-30 88 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/01/2020 Labeler - TriVita, Inc. (128295040)