Label: NITROFURANTOIN- MONOHYDRATE/MACROCRYSTALS capsule
- NDC Code(s): 82868-065-14
- Packager: Northwind Pharmaceuticals, LLC
- This is a repackaged label.
- Source NDC Code(s): 59746-762
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of nitrofurantoin capsules, USP (monohydrate/macrocrystals) and other antibacterial drugs, nitrofurantoin ...
-
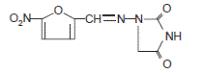
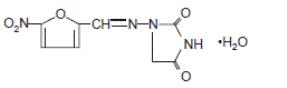
DESCRIPTIONNitrofurantoin is an antibacterial agent specific for urinary tract infections. Nitrofurantoin capsule, USP (monohydrate/macrocrystals) is a hard gelatin capsule. Each capsule contains the ...
-
CLINICAL PHARMACOLOGYEach nitrofurantoin capsule (monohydrate/macrocrystals) contains two forms of nitrofurantoin. Twenty-five percent is macrocrystalline nitrofurantoin, which has slower dissolution and absorption ...
-
INDICATIONS AND USAGENitrofurantoin capsules (monohydrate/macrocrystals) are indicated only for the treatment of acute uncomplicated urinary tract infections (acute cystitis) caused by susceptible strains of ...
-
CONTRAINDICATIONSAnuria, oliguria, or significant impairment of renal function (creatinine clearance under 60 mL per minute or clinically significant elevated serum creatinine) are contraindications. Treatment of ...
-
WARNINGSPulmonary reactions: ACUTE, SUBACUTE, OR CHRONIC PULMONARY REACTIONS HAVE BEEN OBSERVED IN PATIENTS TREATED WITH NITROFURANTOIN. IF THESE REACTIONS OCCUR,NITROFURANTOIN SHOULD BE DISCONTINUED ...
-
PRECAUTIONSInformation for Patients - Patients should be advised to take nitrofurantoin capsules (monohydrate/macrocrystals) with food (ideally breakfast and dinner) to further enhance tolerance and improve ...
-
ADVERSE REACTIONSIn clinical trials of nitrofurantoin, the most frequent clinical adverse events that were reported as possibly or probably drug-related were nausea (8%), headache (6%), and flatulence (1.5%) ...
-
OVERDOSAGEOccasional incidents of acute overdosage of nitrofurantoin have not resulted in any specific symptoms other than vomiting. Induction of emesis is recommended. There is no specific antidote, but a ...
-
DOSAGE AND ADMINISTRATIONNitrofurantoin capsules (monohydrate/macrocrystals), should be taken with food. Adults and Pediatric Patients Over 12 Years:One 100 mg capsule every 12 hours for Seven days.
-
HOW SUPPLIEDNitrofurantoin capsules, USP (monohydrate/macrocrystals), 100 mg, are supplied as a Light yellow to yellow powder blend and yellow colored tablet filled in the hard gelatin capsule shell size '1 ...
-
CLINICAL STUDIESControlled clinical trials comparing nitrofurantoin 100 mg p.o. q12h and nitrofurantoin macrocrystals 50 mg p.o. q6h in the treatment of acute uncomplicated urinary tract infections demonstrated ...
-
Principal Display PanelNDC: 82868-065-14
-
INGREDIENTS AND APPEARANCEProduct Information