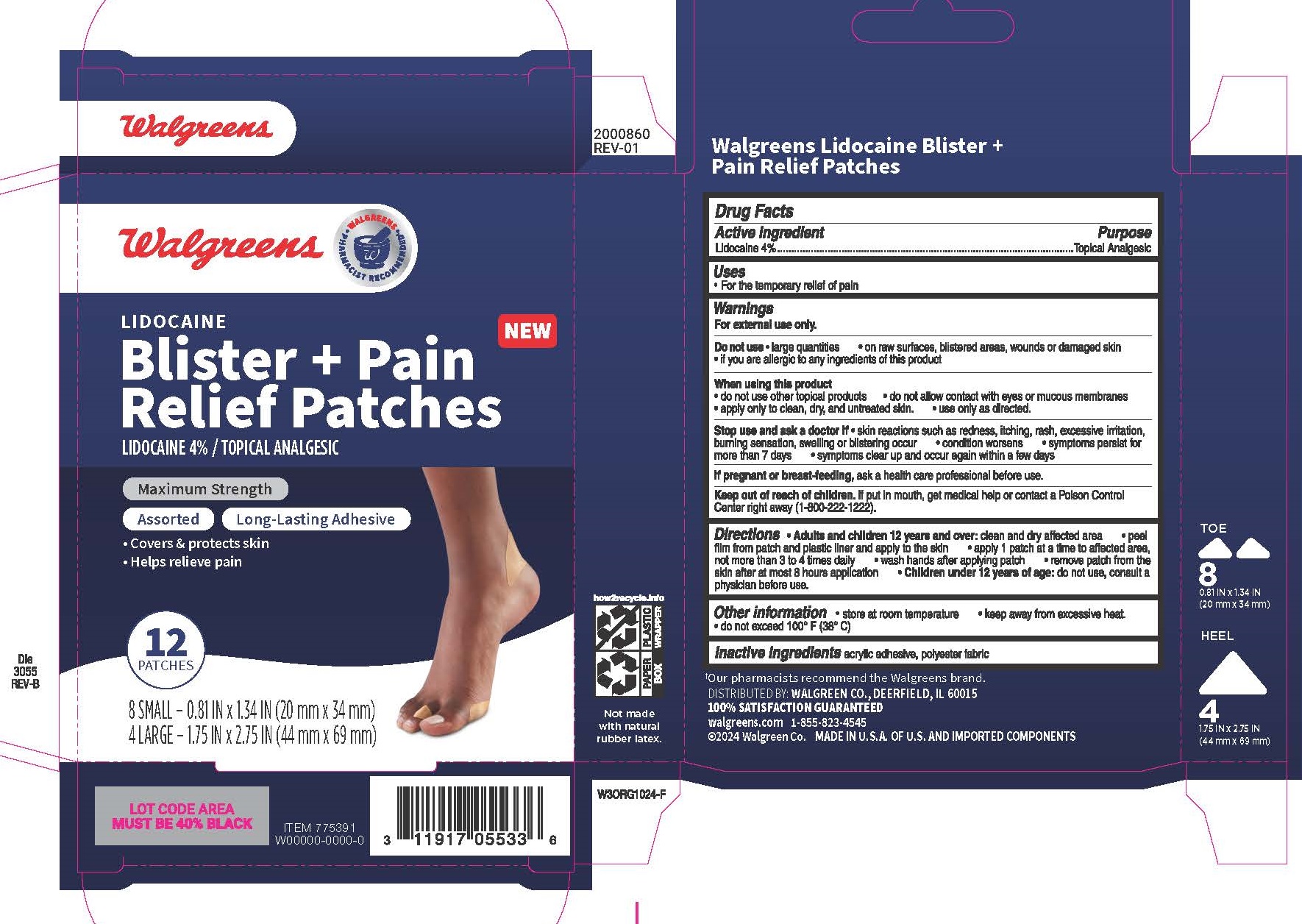
Label: WALGREENS- lidocaine patch
- NDC Code(s): 0363-1002-40
- Packager: WALGREENS
- This is a repackaged label.
- Source NDC Code(s): 65604-100
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient
Lidocaine 4%
-
Purpose
Topical Analgesic
-
Uses
For the temporary relief of pain
-
Warnings
For external use only - Do not use - large quantities - on raw surfaces, blistered areas, wounds or damaged skin - if you are allergic to any ingredients of this product - When using this ...
-
Directions
Adults and children 12 years and over: clean and dry affected area - peel film from patch and plastic liner and apply to the skin - apply 1 patch at a time to affected area, not more than 3 to 4 ...
-
Other information
Store at room temperature - keep away from excessive heat - do not exceed 100 - oF (38 - oC)
-
Inactive ingredients
acrylic adhesive, polyester fabric
-
Principal Display Panel
Walgreens - LIDOCAINE - Blister + Pain Relief Patches - LIDOCAINE 4% / TOPICAL ANALGESIC - Maximum Strength - Assorted Long-Lasting Adhesive - Covers & protects skin - Helps relieve pain - 12 ...
-
INGREDIENTS AND APPEARANCEProduct Information