Label: BLISTEX MEDICATED LIP BALM VARIETY PACK (BLISTEX MEDICATED BERRY LIP BALM, BLISTEX MEDICATED LIP BALM, BLISTEX MEDICATED MINT LIP BALM)- dimethicone, octinoxate, and octisalate kit
- NDC Code(s): 10157-9908-1
- Packager: Blistex Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
beeswax, camphor, cetyl alcohol, cetyl palmitate, euphorbia cerifera (candelilla) wax, flavors, isopropyl myristate, lanolin, lanolin oil, menthol, methyl salicylate1, mineral oil, ozokerite, paraffin, petrolatum, phenoxyethanol, polybutene, red 6 lake1, theobroma cacao (cocoa) seed butter, titanium dioxide
- 1
- may contain these ingredients
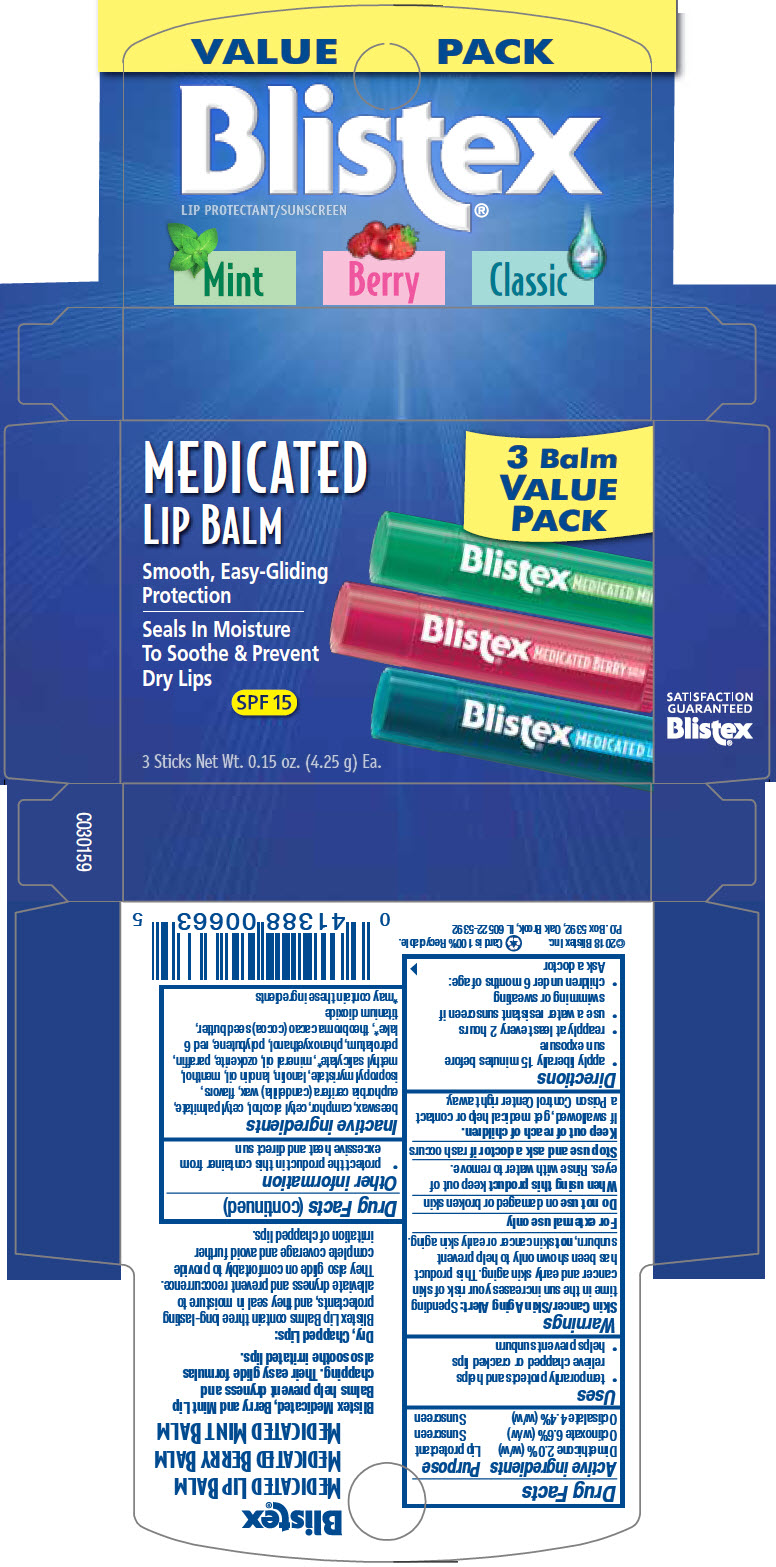
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
BLISTEX MEDICATED LIP BALM VARIETY PACK (BLISTEX MEDICATED BERRY LIP BALM, BLISTEX MEDICATED LIP BALM, BLISTEX MEDICATED MINT LIP BALM)
dimethicone, octinoxate, and octisalate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10157-9908 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10157-9908-1 1 in 1 CARTON 02/03/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 CYLINDER 4.25 g Part 2 1 CYLINDER 4.25 g Part 3 1 CYLINDER 4.25 g Part 1 of 3 BLISTEX MEDICATED BERRY LIP BALM
dimethicone, octinoxate, and octisalate stickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 2 g in 100 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.6 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.4 g in 100 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) CERESIN (UNII: Q1LS2UJO3A) CANDELILLA WAX (UNII: WL0328HX19) LANOLIN OIL (UNII: OVV5IIJ58F) YELLOW WAX (UNII: 2ZA36H0S2V) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LANOLIN (UNII: 7EV65EAW6H) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) CETYL PALMITATE (UNII: 5ZA2S6B08X) CETYL ALCOHOL (UNII: 936JST6JCN) PARAFFIN (UNII: I9O0E3H2ZE) COCOA BUTTER (UNII: 512OYT1CRR) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.,.ALPHA.-DIBROMO-D-CAMPHOR (UNII: F89Z8SAG3O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 6 (UNII: 481744AI4O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4.25 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 02/03/2020 Part 2 of 3 BLISTEX MEDICATED LIP BALM
dimethicone, octinoxate, and octisalate stickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 2 g in 100 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.6 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.4 g in 100 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) CERESIN (UNII: Q1LS2UJO3A) CANDELILLA WAX (UNII: WL0328HX19) LANOLIN OIL (UNII: OVV5IIJ58F) YELLOW WAX (UNII: 2ZA36H0S2V) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LANOLIN (UNII: 7EV65EAW6H) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) CETYL PALMITATE (UNII: 5ZA2S6B08X) CETYL ALCOHOL (UNII: 936JST6JCN) PARAFFIN (UNII: I9O0E3H2ZE) COCOA BUTTER (UNII: 512OYT1CRR) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.,.ALPHA.-DIBROMO-D-CAMPHOR (UNII: F89Z8SAG3O) METHYL SALICYLATE (UNII: LAV5U5022Y) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 6 (UNII: 481744AI4O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4.25 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 02/03/2020 Part 3 of 3 BLISTEX MEDICATED MINT LIP BALM
dimethicone, octinoxate, and octisalate ointmentProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 2 g in 100 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.6 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.4 g in 100 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) CERESIN (UNII: Q1LS2UJO3A) CANDELILLA WAX (UNII: WL0328HX19) LANOLIN OIL (UNII: OVV5IIJ58F) YELLOW WAX (UNII: 2ZA36H0S2V) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LANOLIN (UNII: 7EV65EAW6H) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) CETYL PALMITATE (UNII: 5ZA2S6B08X) CETYL ALCOHOL (UNII: 936JST6JCN) PARAFFIN (UNII: I9O0E3H2ZE) COCOA BUTTER (UNII: 512OYT1CRR) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.,.ALPHA.-DIBROMO-D-CAMPHOR (UNII: F89Z8SAG3O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4.25 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 02/03/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 02/03/2020 Labeler - Blistex Inc (005126354) Establishment Name Address ID/FEI Business Operations Blistex Inc 005126354 MANUFACTURE(10157-9908)