Label: ESZOPICLONE tablet, film coated
- NDC Code(s): 0093-5537-56, 0093-5538-01, 0093-5539-01
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ESZOPICLONE TABLETS safely and effectively. See full prescribing information for ESZOPICLONE TABLETS. ESZOPICLONE tablets, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: COMPLEX SLEEP BEHAVIORS
Complex sleep behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur following use of eszopiclone tablets. Some of these events may result in serious injuries, including death. Discontinue eszopiclone tablets immediately if a patient experiences a complex sleep behavior [see Contraindications (4) and Warnings and Precautions (5.1)].
Close
-
1 INDICATIONS AND USAGEEszopiclone tablets are indicated for the treatment of insomnia. In controlled outpatient and sleep laboratory studies, eszopiclone tablets administered at bedtime decreased sleep latency and ...
-
2 DOSAGE AND ADMINISTRATIONUse the lowest effective dose for the patient. 2.1 Dosage in Adults - The recommended starting dose is 1 mg. Dosing can be raised to 2 mg or 3 mg if clinically indicated. In some patients, the ...
-
3 DOSAGE FORMS AND STRENGTHSEszopiclone Tablets, USP are available in 1 mg, 2 mg and 3 mg strengths for oral administration. Eszopiclone 1 mg tablets are light-blue, film-coated, unscored, convex, round tablets, debossed ...
-
4 CONTRAINDICATIONSEszopiclone tablets is contraindicated in patients who have experienced complex sleep behaviors after taking eszopiclone tablets [see Warnings and Precautions (5.1)]. Eszopiclone tablets are ...
-
5 WARNINGS AND PRECAUTIONS5.1 Complex Sleep Behaviors - Complex sleep behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur following the first or any ...
-
6 ADVERSE REACTIONSThe following are described in more detail in the Warnings and Precautions section of the label: Complex Sleep Behaviors [see Boxed Warning and Warnings and Precautions (5.1)] CNS Depressant ...
-
7 DRUG INTERACTIONS7.1 CNS Active Drugs - Ethanol: An additive effect on psychomotor performance was seen with coadministration of eszopiclone and ethanol [see Warnings and Precautions (5.1, 5.2)]. Olanzapine ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available pharmacovigilance data with eszopiclone use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage, or ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Eszopiclone is a Schedule IV controlled substance under the Controlled Substances Act. Other substances under the same classification are benzodiazepines and the ...
-
10 OVERDOSAGEIn clinical trials with eszopiclone, one case of overdose with up to 36 mg of eszopiclone was reported in which the subject fully recovered. Since commercial marketing began, spontaneous cases of ...
-
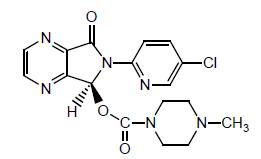
11 DESCRIPTIONEszopiclone, USP is a nonbenzodiazepine hypnotic agent that is a pyrrolopyrazine derivative of the cyclopyrrolone class. The chemical name of eszopiclone, USP is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of eszopiclone as a hypnotic is unclear; however, its effect could be related to its interaction with GABA-receptor complexes at binding domains ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a carcinogenicity study in rats, oral administration of eszopiclone for 97 (males) or 104 (females) weeks resulted ...
-
14 CLINICAL STUDIESThe effect of eszopiclone on reducing sleep latency and improving sleep maintenance was established in studies with 2100 subjects (ages 18 to 86) with chronic and transient insomnia in six ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEszopiclone Tablets, USP are available as follows: Eszopiclone 1 mg tablets are light-blue, film-coated, unscored, convex, round tablets, debossed “93” on one side of the tablet and “E7” on the ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Medication Guide). Inform patients and their families about the benefits and risks of treatment with eszopiclone. Inform patients of the availability of a ...
-
MEDICATION GUIDEDispense with Medication Guide available at: www.tevausa.com/medguides. Eszopiclone (es zoe' pi klone) Tablets, Coated CIV - Read the Medication Guide that comes with eszopiclone tablets before ...
-
Package/Label Display PanelNDC 0093-5537-56 - Eszopiclone Tablets 1 mg - CIV - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 30 TABLETS
-
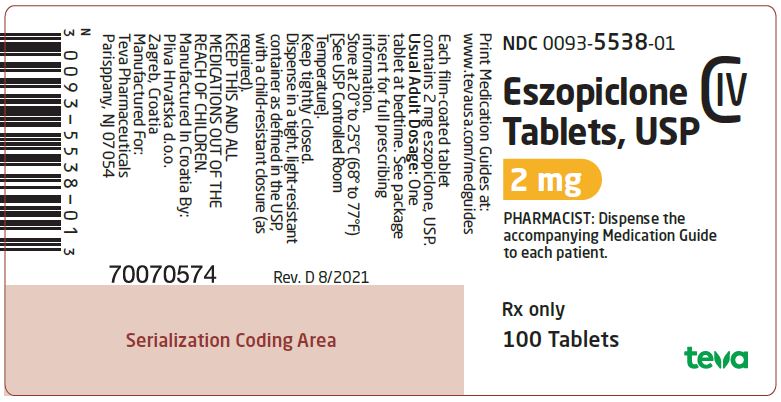
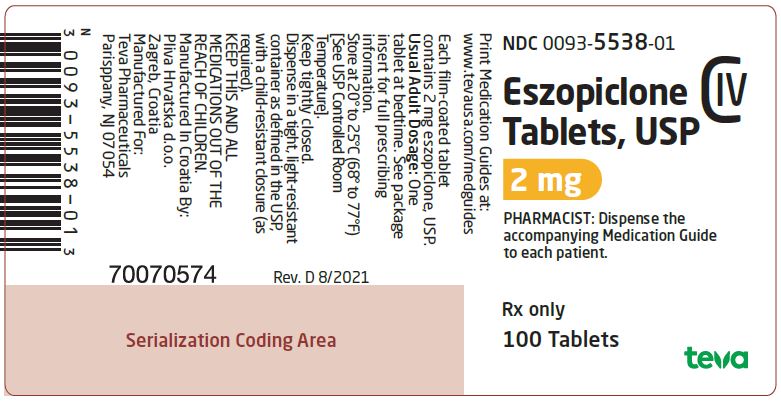
Package/Label Display PanelNDC 0093-5538-01 - Eszopiclone Tablets 2 mg - CIV - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 100 TABLETS
-
Package/Label Display PanelNDC 0093-5539-01 - Eszopiclone Tablets 3 mg - CIV - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx only - 100 TABLETS
-
INGREDIENTS AND APPEARANCEProduct Information