Label: BENZTROPINE MESYLATE tablet
- NDC Code(s): 70518-2732-0, 70518-2732-1, 70518-2732-2
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 69315-136
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
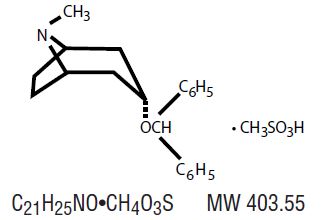
DESCRIPTIONBenztropine mesylate, USP is a synthetic compound containing structural features found in atropine and diphenhydramine. It is designated chemically as 3α-(Diphenylmethoxy) -1α-H,5 α H-tropane ...
-
CLINICAL PHARMACOLOGYBenztropine mesylate possesses both anticholinergic and antihistaminic effects, although only the former have been established as therapeutically significant in the management of parkinsonism. In ...
-
INDICATIONS AND USAGEBenztropine mesylate tablets, USP are indicated for use as an adjunct in the therapy of all forms of parkinsonism. Useful also in the control of extrapyramidal disorders (except tardive dyskinesia ...
-
CONTRAINDICATIONSHypersensitivity to benztropine mesylate tablets or to any component of the tablets. Because of its atropine-like side effects, this drug is contraindicated in pediatric patients ...
-
WARNINGSSafe use in pregnancy has not been established. Benztropine mesylate may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating ...
-
PRECAUTIONS
General
Since benztropine mesylate has cumulative action, continued supervision is advisable. Patients with a tendency to tachycardia and patients with prostatic hypertrophy should be observed closely ...
-
Drug Interactions
Antipsychotic drugs such as phenothiazines or haloperidol; tricyclic antidepressants (see - WARNINGS).
-
Pediatric Use
Because of the atropine-like side effects, benztropine mesylate should be used with caution in pediatric patients over three years of age (see - CONTRAINDICATIONS).
-
ADVERSE REACTIONSThe adverse reactions below, most of which are antichlolinergic in nature, have been reported and within each category are listed in order of decreasing severity. Cardiovascular - Tachycardia ...
-
OVERDOSAGEManifestations - May be any of those seen in atropine poisoning or antihistamine overdosage: CNS depression, preceded or followed by stimulation; confusion; nervousness; listlessness ...
-
DOSAGE AND ADMINISTRATIONBenztropine mesylate tablets should be used when patients are able to take oral medication. The injection is especially useful for psychotic patients with acute dystonic reactions or other ...
-
HOW SUPPLIEDBenztropine Mesylate Tablets, USP, for oral use, are supplied in the following forms: As 0.5 mg: White color, round, flat face beveled edge, bisected, compressed tablets, debossed "EP” above the ...
-
PRINCIPAL DISPLAY PANELDRUG: BENZTROPINE MESYLATE - GENERIC: BENZTROPINE MESYLATE - DOSAGE: TABLET - ADMINSTRATION: ORAL - NDC: 70518-2732-0 - NDC: 70518-2732-1 - NDC: 70518-2732-2 - COLOR: white - SHAPE: ROUND - SCORE: Two even ...
-
INGREDIENTS AND APPEARANCEProduct Information