Label: CLEARASIL RAPID RESCUE DEEP TREATMENT PADS- salicylic acid cloth
- NDC Code(s): 63824-431-90
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
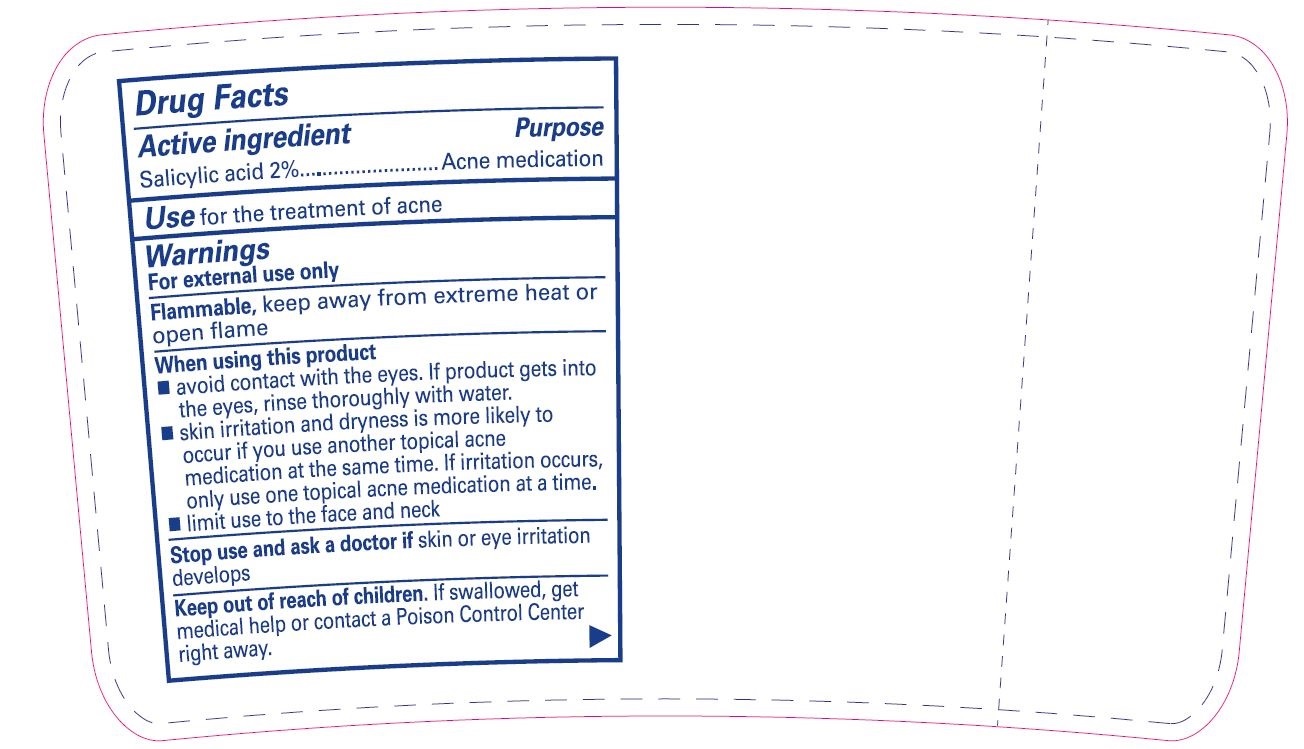
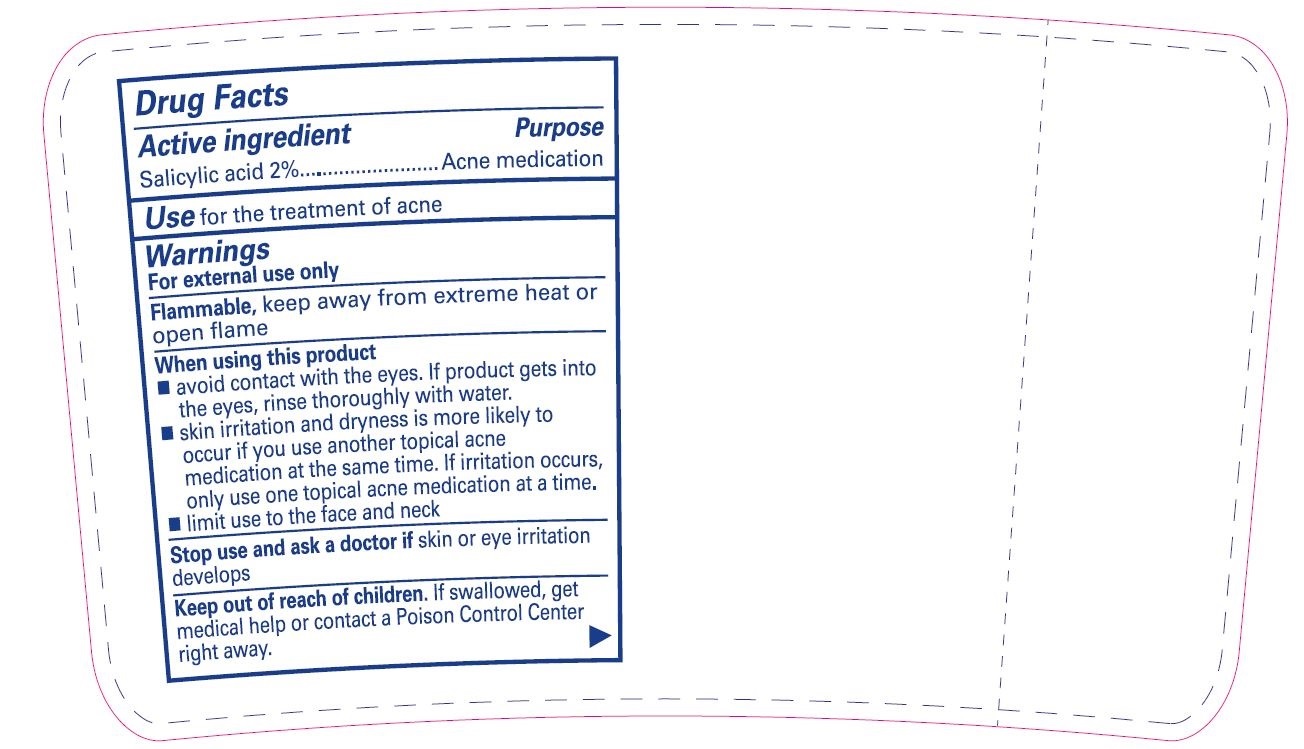
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Flammable,keep away from extreme heat or open flame
When using this product
- avoid contact with the eyes. If product gets into the eyes, rinse thoroughly with water.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- limit use to the face and neck
-
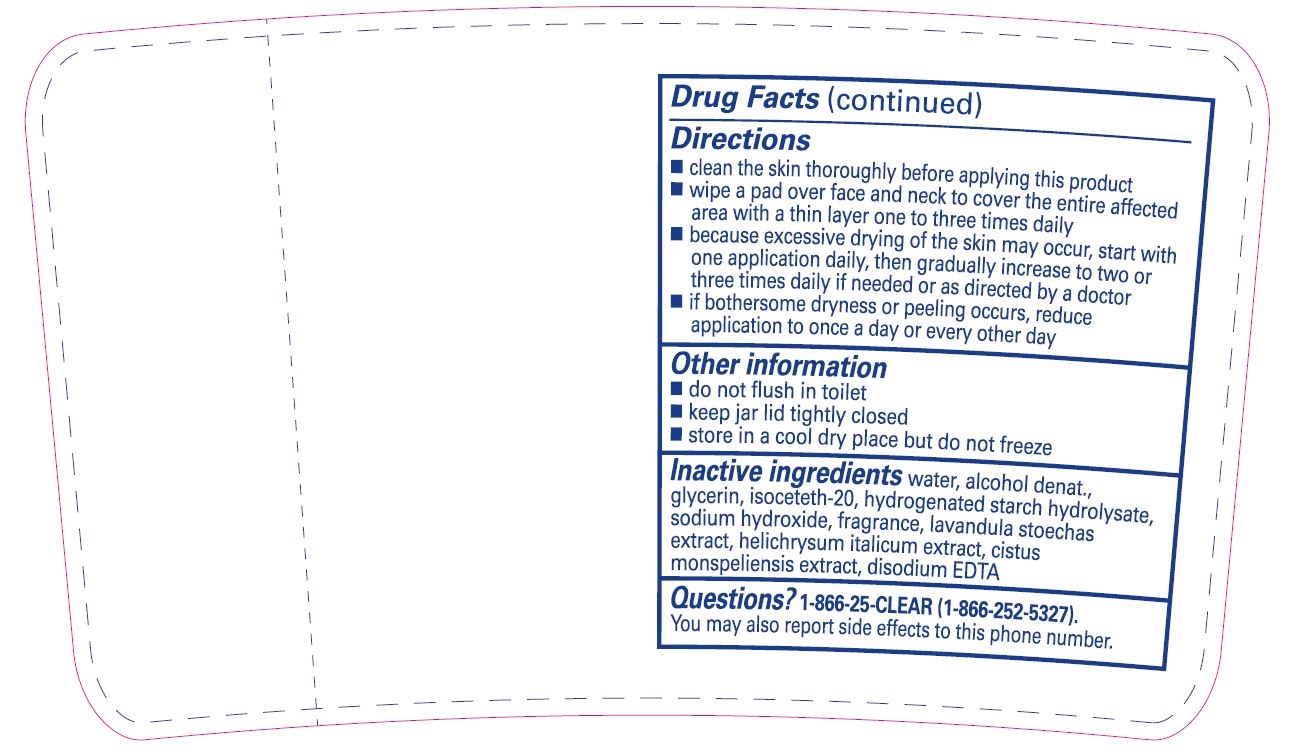
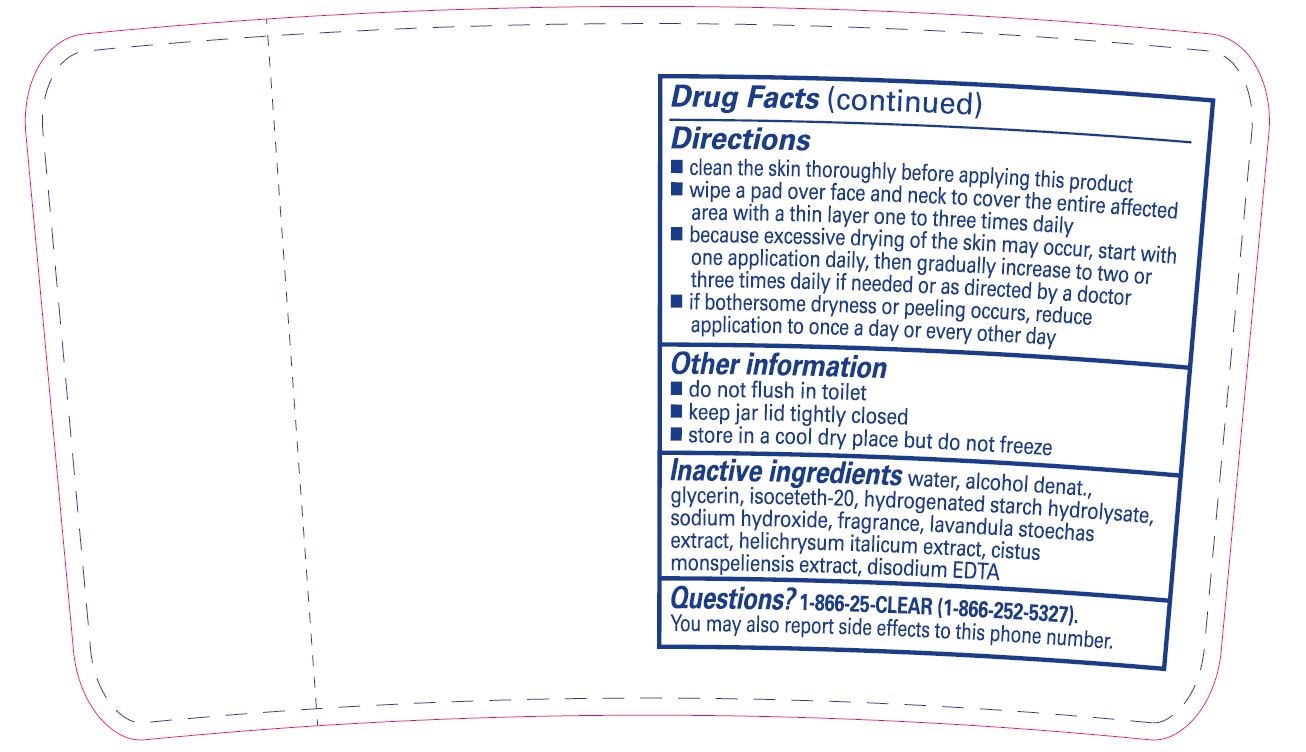
Directions
- clean the skin thoroughly before applying this product
- wipe a pad over face and neck to cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 90 Pad Jar Label
-
INGREDIENTS AND APPEARANCE
CLEARASIL RAPID RESCUE DEEP TREATMENT PADS
salicylic acid clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63824-431 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.02 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) ISOCETETH-20 (UNII: O020065R7Z) HYDROGENATED STARCH HYDROLYSATE (UNII: 27F77DSJ5V) SODIUM HYDROXIDE (UNII: 55X04QC32I) HELICHRYSUM ITALICUM FLOWER (UNII: P62Y550X24) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Product Characteristics Color Score Shape ROUND Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63824-431-90 90 in 1 JAR; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug 12/06/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 12/05/2017 Labeler - RB Health (US) LLC (081049410)