Label: METHYLPREDNISOLONE tablet
- NDC Code(s): 67296-0420-1, 67296-0420-2
- Packager: Redpharm Drug
- This is a repackaged label.
- Source NDC Code(s): 59746-001
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
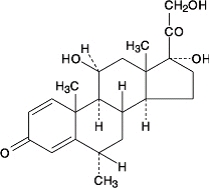
DESCRIPTIONMethylprednisolone Tablets, USP contain methylprednisolone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily ...
-
CLINICAL PHARMACOLOGYNaturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic ...
-
INDICATIONS AND USAGEMethylprednisolone Tablets are indicated in the following conditions: 1.Endocrine Disorders - Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice ...
-
CONTRAINDICATIONSSystemic fungal infections and known hypersensitivity to components.
-
WARNINGSIn patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is ...
-
PRECAUTIONSGeneral Precautions - Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after ...
-
ADVERSE REACTIONSFluid and Electrolyte Disturbances - Sodium retention - Congestive heart failure in susceptible patients - Hypertension - Fluid retention - Potassium loss - Hypokalemic alkalosis - Musculoskeletal - Muscle ...
-
DOSAGE AND ADMINISTRATIONThe initial dosage of Methylprednisolone Tablets may vary from 4 mg to 48 mg of methylprednisolone per day depending on the specific disease entity being treated. In situations of less severity ...
-
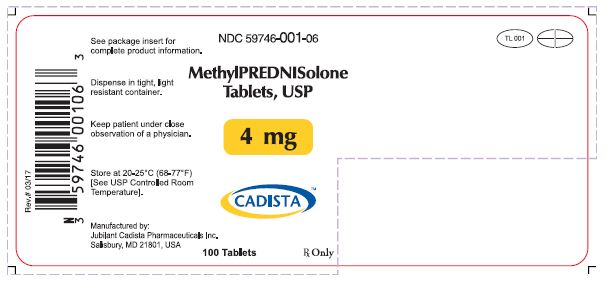
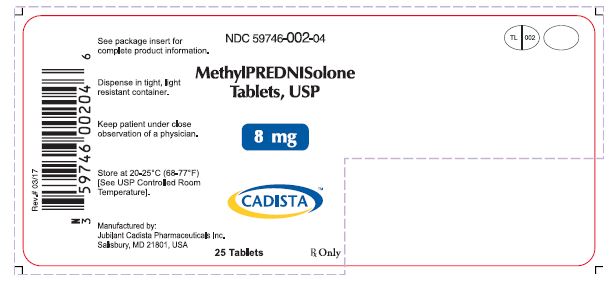
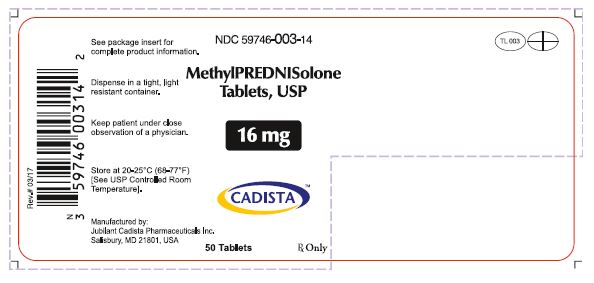
HOW SUPPLIEDMethylprednisolone Tablets, USP are available in the following strengths and package sizes: 4mg(White, oval shaped tablets, debossed with "TL 001" on one side and quadrisected on the other ...
-
PACKAGE LABELNDC 59746- 001-06 - MethylPREDNISolone Tablets, USP - 4 mg - CADISTA™ 100 Tablets - Rx Only - Rev # 03/17 - See package insert for - complete ...
-
INGREDIENTS AND APPEARANCEProduct Information