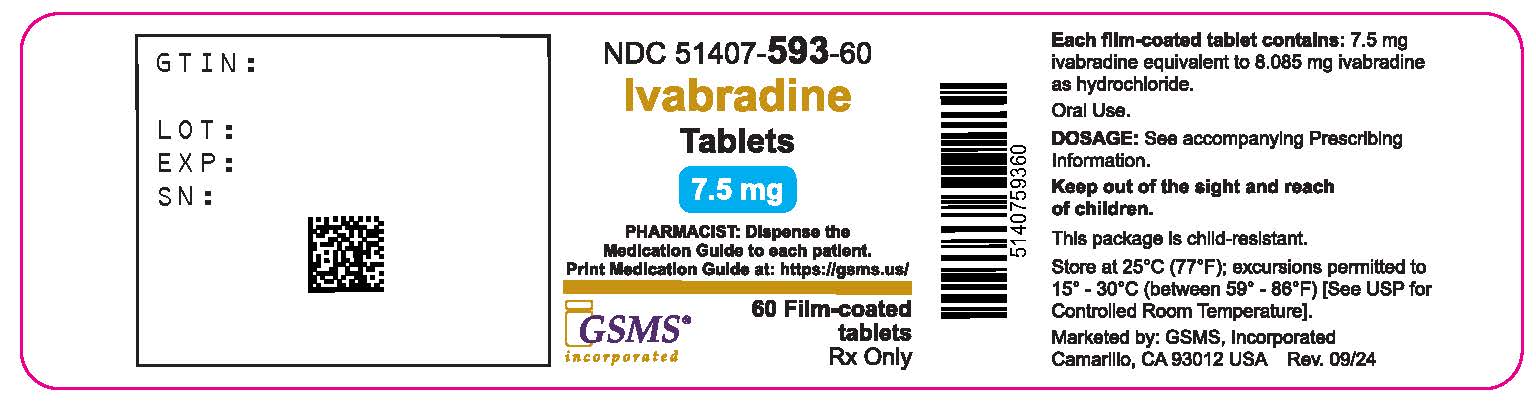
Label: IVABRADINE tablet, film coated
- NDC Code(s): 51407-592-60, 51407-593-60
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 50742-362, 50742-363
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 15, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONIVABRADINE TABLETS. These highlights do not include all the information needed to use IVABRADINE TABLETS safely and effectively. See full prescribing information for IVABRADINE TABLETS - IVABRADINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Heart Failure in Adult Patients - Ivabradine tablets are indicated to reduce the risk of hospitalization for worsening heart failure in adult patients with stable, symptomatic chronic heart ...
-
2 DOSAGE AND ADMINISTRATION2.1 Adults - The recommended starting dose of ivabradine tablets is 5 mg twice daily with food. Assess patient after two weeks and adjust dose to achieve a resting heart rate between 50 and 60 ...
-
3 DOSAGE FORMS AND STRENGTHSTablets - Ivabradine tablets 5 mg: Light salmon-colored, capsule-shaped, film-coated scored tablet debossed with "I5" divided by score on one side and plain on other side. The tablet is scored and ...
-
4 CONTRAINDICATIONSIvabradine tablets are contraindicated in patients with: Acute decompensated heart failure - Clinically significant hypotension - Sick sinus syndrome, sinoatrial block or 3 - rddegree AV block ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Ivabradine tablets may cause fetal toxicity when administered to a pregnant woman based on findings in animal studies. Embryo-fetal toxicity and cardiac teratogenic effects ...
-
6 ADVERSE REACTIONSClinically significant adverse reactions that appear in other sections of the labeling include: Atrial Fibrillation - [see - Warnings and Precautions (5.2)] Bradycardia and ...
-
7 DRUG INTERACTIONS7.1 Cytochrome P450-Based Interactions - Ivabradine is primarily metabolized by CYP3A4. Concomitant use of CYP3A4 inhibitors increases ivabradine plasma concentrations and use of CYP3A4 inducers ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animals, ivabradine tablets may cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of ...
-
10 OVERDOSAGEOverdose may lead to severe and prolonged bradycardia. In the event of bradycardia with poor hemodynamic tolerance, temporary cardiac pacing may be required. Supportive treatment, including ...
-
11 DESCRIPTIONIvabradine tablets contains ivabradine as the active pharmaceutical ingredient. Ivabradine is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that reduces the spontaneous ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ivabradine tablets blocks the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel responsible for the cardiac pacemaker I - fcurrent, which regulates ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There was no evidence of carcinogenicity when mice and rats received ivabradine up to 104 weeks by dietary administration. High doses ...
-
14 CLINICAL STUDIES14.1 Heart Failure in Adult Patients - SHIFT - The Systolic Heart failure treatment with the I - finhibitor ivabradine Trial (SHIFT) was a randomized, double-blind trial comparing ivabradine ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGIvabradine tablets 5 mg tablets are formulated as Light salmon-colored, Capsule-shaped, film-coated scored tablets debossed with "I5" divided by the score on one side and plain on the other. They ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labelling - [see Medication Guide and Instructions for Use]. ● Fetal Toxicity - Advise pregnant women of the potential risks to a ...
-
SPL MEDGUIDEDispense with Medication Guide available at: www.ingenus.com/medguide/ivabradine-tablets.pdf - This Medication Guide has been approved by the U.S. Food and Drug ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information