Label: TORSEMIDE- torsemide tablet
- NDC Code(s): 72865-258-90, 72865-259-01, 72865-259-90, 72865-260-01, view more
- Packager: XLCare Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TORSEMIDE TABLETS safely and effectively. See full prescribing information for TORSEMIDE TABLETS - TORSEMIDE tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Edema - Torsemide tablets are indicated for the treatment of edema associated with heart failure, renal disease or hepatic disease. 1.2 Hypertension - Torsemide tablets are indicated for ...
-
2 DOSAGE AND ADMINISTRATION2.1 Treatment of Edema - Edema associated with heart failure - The recommended initial dose is 10 mg or 20 mg oral torsemide tablets once daily. If the diuretic response is inadequate ...
-
3 DOSAGE FORMS AND STRENGTHSTorsemide tablets USP, 5 mg are available as white to off white, oval shaped, scored tablets, debossed with ‘56’ on scored side and ‘H’ on the opposite side. Torsemide tablets USP, 10 mg ...
-
4 CONTRAINDICATIONSTorsemide tablets are contraindicated in patients with known hypersensitivity to torsemide tablets or to povidone. Torsemide tablets are contraindicated in patients who are anuric ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension and Worsening Renal Function - Excessive diuresis may cause potentially symptomatic dehydration, blood volume reduction and hypotension and worsening renal function, including ...
-
6 ADVERSE REACTIONSThe following risks are discussed in more detail in others sections: • Hypotension and Worsening Renal Function - [see Warnings and Precautions - (5.1)] • Electrolyte and ...
-
7 DRUG INTERACTIONS7.1 Nonsteroidal Anti-inflammatory Drugs - Because torsemide and salicylates compete for secretion by renal tubules, patients receiving high doses of salicylates may experience salicylate ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on use of torsemide in pregnant women and the risk of major birth defects or miscarriage. In pregnant rats and rabbits dosed, on a ...
-
10 OVERDOSEThe signs and symptoms of overdosage can be anticipated to include those of excessive pharmacologic effect: dehydration, hypovolemia, hypotension, hyponatremia, hypokalemia, hypochloremic ...
-
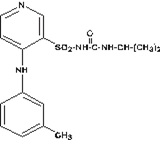
11 DESCRIPTIONTorsemide is a diuretic of the pyridine-sulfonylurea class. Its chemical name is 1-isopropyl-3-[(4-m-toluidino-3-pyridyl) sulfonyl]urea and its structural formula is: Its molecular ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Micropuncture studies in animals have shown that torsemide acts from within the lumen of the thick ascending portion of the loop of Henle, where it inhibits the Na ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility - No overall increase in tumor incidence was found when torsemide was given to rats and mice throughout their lives at doses up to 9 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTorsemide tablets USP, 5 mg are white to off white, oval shaped, scored tablets, debossed with ‘56’ on scored side and ‘H’ on the opposite side. They are supplied as follows: Bottle of 90 ...
-
17 PATIENT COUNSELING INFORMATIONSymptomatic Hypotension:Advise patients receiving torsemide tablets that lightheadedness can occur, especially during the first days of therapy, and that it should be reported to the ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELTorsemide Tablets, 5 mg -90s count - Torsemide Tablets, 10 mg -100s count - Torsemide Tablets, 20 mg -100s count - Torsemide Tablets, 100 mg -100s count
-
INGREDIENTS AND APPEARANCEProduct Information