Label: TETROXY HCA- oxytetracycline hcl powder
- NDC Code(s): 61133-5010-1, 61133-5010-2, 61133-5010-3
- Packager: Bimeda, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Tetroxy® HCA-1400
(oxytetracycline hydrochloride soluble powder)
Antibiotic
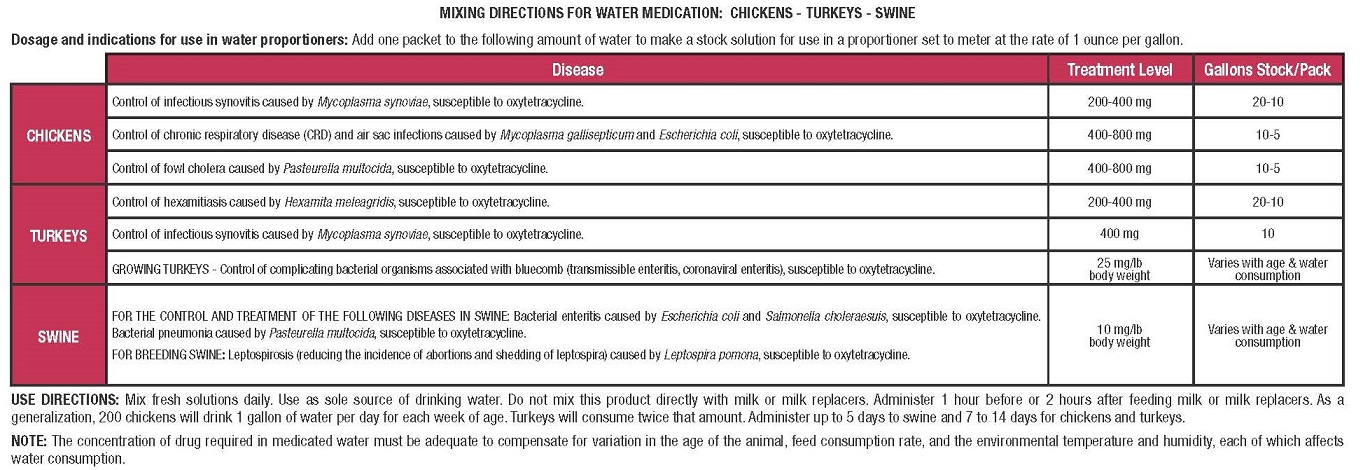
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.This packet contains 512 g of oxytetracycline hydrochloride and will make:
2560 gallons (9690 liters) containing 200 mg oxytetracycline hydrochloride per gallon
1280 gallons (4845 liters) containing 400 mg oxytetracycline hydrochloride per gallon
640 gallons (2422 liters) containing 800 mg oxytetracycline hydrochloride per gallon
This packet will treat 51,200 lbs of swine.
This packet will treat 20,480 lbs of turkey at 25 mg/lb body weight.For Use in Drinking Water Only
Not For Use in Liquid Medicated Feeds
Not For Use in Humans
Keep Out of Reach of Children
HAZARDOUS REMEDY
See back panel for instructions.
Approved by FDA under ANADA # 200-144Net Weight: 3.09 lb (1400 g)
- DOSAGE & ADMINISTRATION
- PRECAUTIONS
- RESIDUE WARNING
-
STORAGE AND HANDLING
RECOMMENDED PACKET STORAGE CONDITIONS: Store between 20°C - 25°C (68°F - 77°F) with excursions permitted between 15°C - 40°C (59°F - 104°F).
CONTACT INFORMATION: To report adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TETROXY HCA
oxytetracycline hcl powderProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:61133-5010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYTETRACYCLINE HYDROCHLORIDE (UNII: 4U7K4N52ZM) (OXYTETRACYCLINE ANHYDROUS - UNII:SLF0D9077S) OXYTETRACYCLINE HYDROCHLORIDE 648 g in 1772 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61133-5010-1 280 g in 1 POUCH 2 NDC:61133-5010-2 1400 g in 1 POUCH 3 NDC:61133-5010-3 1772 g in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200144 07/02/2004 Labeler - Bimeda, Inc. (060492923) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda, Inc. 060492923 manufacture