1-DOSE TREATMENT
-
Tioconazole Ointment 6.5%
VAGINAL ANTIFUNGAL
-
EDUCATIONAL BROCHURE
-
Please read this educational brochure completely before using this product.
USE: treats vaginal yeast ...
1-DOSE TREATMENT
Tioconazole Ointment 6.5%
VAGINAL ANTIFUNGAL
EDUCATIONAL BROCHURE
Please read this educational brochure completely before using this product.
USE: treats vaginal yeast infections
1 THIS PRODUCT CURES MOST RECURRENT VAGINAL YEAST INFECTIONS
IF THIS IS THE FIRST TIME YOU HAVE HAD VAGINAL ITCH AND DISCOMFORT, CONSULT YOUR DOCTOR. IF YOU HAVE HAD A DOCTOR DIAGNOSE A VAGINAL YEAST INFECTION BEFORE AND HAVE THE SAME SYMPTOMS NOW, USE THIS OINTMENT AS DIRECTED. THIS PRODUCT IS FOR THE TREATMENT OF RECURRENT VAGINAL YEAST INFECTIONS ONLY. IT DOES NOT TREAT OTHER INFECTIONS AND DOES NOT PREVENT PREGNANCY.
This product, a vaginal ointment that contains an antifungal medicine, works to cure most recurrent vaginal yeast infections by killing the overgrowth of yeast.
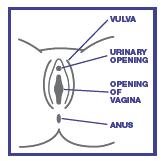
2 WHAT IS A VAGINAL YEAST INFECTION (CANDIDIASIS)?
A yeast infection is caused by an organism called Candida, which is a type of yeast. Healthy women usually have this yeast in the vagina. Either an overgrowth or rapid growth of this yeast can cause a vaginal yeast infection. The symptoms of a vaginal yeast infection can include any or all of the following:
- •
-
Vaginal itching – Itching can range from mild to intense.
- •
-
Vaginal discharge – This discharge may be thick like paste or lumpy like cottage cheese.
- •
-
Vaginal soreness, burning or irritation – The vagina can feel sore inside or there can be a burning sensation, particularly during vaginal intercourse.
- •
-
Rash or redness – Rash or redness may occur around the vagina (vulvar irritation).
NOTE: Vaginal discharge that is different from the above, for example, a yellow/green discharge or a discharge that smells "fishy" (foul-smelling), may indicate that you have something other than a yeast infection. If this is the case, you should consult your doctor before using this product.
3 WHAT ARE THE REASONS YOU GET VAGINAL YEAST INFECTIONS?
There are several conditions that can cause too much yeast to grow inside the vagina such as:
- •
-
Hormonal changes – Changes in hormonal levels can occur during pregnancy, with the use of some birth control pills, and just before a woman's menstrual period.
- •
-
Antibiotics – Because antibiotics can kill the normal bacteria in the vagina, yeast may grow very quickly.
- •
-
Diabetes – Diabetes can also make a woman more likely to get a vaginal yeast infection.
- •
-
Human Immunodeficiency Virus (HIV) – Various medical conditions can damage the body's normal defenses against infection. One of the most serious of these conditions is infection with the human immunodeficiency virus (HIV -the virus that causes AIDS). Infection with HIV causes the body to be more susceptible to infections, including vaginal yeast infections. Women with HIV infection may have frequent vaginal yeast infections or, especially, vaginal yeast infections that do not clear up easily with proper treatment. If you may have been exposed to HIV and are experiencing either frequent recurring vaginal yeast infections or, especially, vaginal yeast infections that do not clear up easily with proper treatment, you should see your doctor promptly. If you wish further information on risk factors for HIV infection or on the relationship between recurrent or persistent vaginal yeast infections and HIV infection, please contact your doctor or the CDC National AIDS HOTLINE. The CDC phone numbers are: 1-800-232-4636 (English/Spanish), or 1-888-232-6348 (hearing impaired, TTY).
4 WARNINGS
For vaginal use only
Do not use if you have never had a vaginal yeast infection diagnosed by a doctor
Ask a doctor before use if you have
- •
-
vaginal itching and discomfort for the first time
- •
-
lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- •
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- •
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS
When using this product
- •
- do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted disease (STDs).
- •
- do not have vaginal intercourse
- •
- mild increase in vaginal burning, itching or irritation may occur
- •
- if you do not get complete relief ask a doctor before using another product
Stop use and ask a doctor if
- •
-
symptoms do not get better after 3 days
- •
-
symptoms last more than 7 days
- •
-
you get a rash or hives, abdominal pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222).
5 WHEN CAN YOU EXPECT SYMPTOM RELIEF?
While this product is a 1-dose treatment, most women do not experience complete relief of their symptoms in just 1 day. Most women experience some improvement within 1 day and complete relief of symptoms within 7 days.
If your symptoms do not improve in 3 days or if you still have symptoms after 7 days, consult your doctor. Your doctor may recommend other treatment, or you may have a condition other than a yeast infection.
If your symptoms return within 2 months or if you think you have been exposed to the human immunodeficiency virus (HIV) that causes AIDS, consult your doctor immediately. Recurring yeast infections may be a sign of pregnancy or a serious condition, such as AIDS or diabetes.
6 HOW TO USE TIOCONAZOLE OINTMENT 6.5%
This product comes in a pre-filled, single-dose applicator.
Directions for use:
- •
-
Read the full directions printed below and on the printed packet carefully before using.
- •
- Bedtime is the best time to apply this product.
- •
- Open the printed packet just before use.
- •
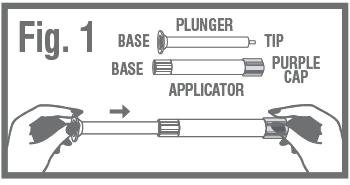
- Remove the applicator and plunger from the printed packet. The applicator is pre-filled with vaginal ointment.
- •
- While firmly holding the purple-capped end of the applicator, push the tip of the plunger into the base of applicator. Fig. 1
- •
-
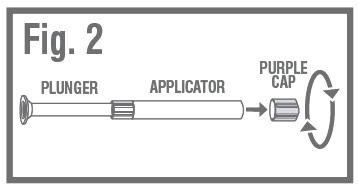
REMOVE PURPLE CAPfrom the top of the applicator using a pull-twist action. Fig. 2
- •
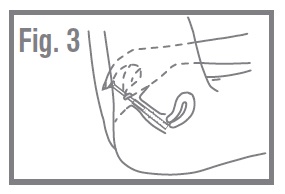
- Lie on your back with your knees bent. Gently insert applicator into the vagina as far as it will go comfortably. Fig. 3
- •
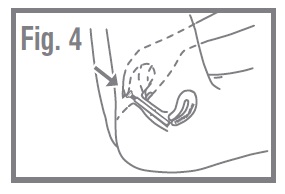
- Push the plunger into the applicator until it will go no farther. Withdraw the applicator and plunger and dispose of it in the wastebasket. Do not flush. Fig. 4
- •
-
DO NOT USE TAMPONS while using this medicine. Use sanitary napkins instead.
7 ADVERSE REACTIONS (SIDE EFFECTS)
A temporary increase in vaginal burning, itching and/or irritation may be experienced upon insertion of this ointment. These reactions, in addition to vaginal swelling or redness; difficulty or burning on urination; headache; abdominal pain/cramping and upper respiratory infection have been reported with the use of this product. If you experience any of these, consult your doctor.
8 COMMON QUESTIONS ABOUT VAGINAL YEAST INFECTIONS AND TIOCONAZOLE OINTMENT 6.5%
Q. Should I use this product while I have my period?
You should use this product while menstruating but you should not use tampons following application. Use sanitary napkins instead.
Q. Can I have sex while using this product?
It is generally recommended not to have vaginal intercourse the first few nights after treatment with this product.
Q. Can I use a condom or diaphragm while using this product?
No. This product contains petrolatum which may damage the latex from which condoms and diaphragms are made and may cause them to fail. This may reduce their effectiveness in preventing sexually transmitted diseases and pregnancy. You should wait 3 days after treatment to resume using condoms or your diaphragm.
Q. Does my partner need to be treated?
Yeast infections occur less frequently in men than in women. However, if your partner has any of the following symptoms: itching, redness or discomfort in the genital area, he should see a doctor and mention that you are treating a vaginal yeast infection.
Q. Can I use feminine hygiene products (such as douche) while I have a vaginal yeast infection?
It is best not to use any vaginal preparations while you have a yeast infection including douches, feminine hygiene sprays, contraceptive foams, inserts, or jellies.
9 FOR BEST RESULTS
- •
-
Wear cotton underwear and loose-fitting clothes – Yeast grow in warm, moist environments. Wearing loose-fitting, cotton clothes helps create a cooler environment, which makes it more difficult for yeast to grow.
- •
-
Change out of a wet bathing suit quickly – Dampness is more likely to encourage the growth of yeast.
- •
-
Use proper hygiene – Wipe from front to rear (away from vagina) after a bowel movement or urination.
- •
-
Discuss with your doctor any medication you are now taking – Certain types of medication can make your vagina more prone to infection.
If you have any other questions or concerns about vaginal yeast infections or of a medical nature, don't hesitate to consult your doctor.
If you have questions or comments about this product, call our toll-free number 1-800-719-9260.
CONTENTS
Active ingredient: Tioconazole 300 mg (6.5% per applicator).
Inactive ingredients: Butylated hydroxyanisole, magnesium aluminum silicate and white petrolatum.
Store at 20°-25°C (68°-77°F).
Do not use if printed packet is torn, open, or incompletely sealed.
For expiration date and lot number, see carton end flap and applicator printed packet.
Distributed By
Perrigo®
Allegan, MI 49010
: 42600 00 J4
Close