Label: VOXZOGO 0.4MG- vosoritide kit
VOXZOGO 0.56MG- vosoritide kit
VOXZOGO 1.2MG- vosoritide kit
-
NDC Code(s):
68135-061-00,
68135-070-12,
68135-082-36,
68135-094-84, view more68135-102-43, 68135-119-66, 68135-130-92, 68135-158-17, 68135-181-93
- Packager: BioMarin Pharmaceutical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VOXZOGO safely and effectively. See full prescribing information for VOXZOGO.
VOXZOGO (vosoritide) for injection, for subcutaneous use
Initial U.S. Approval: 2021INDICATIONS AND USAGE
VOXZOGO is a C type natriuretic peptide (CNP) analog indicated to increase linear growth in pediatric patients with achondroplasia with open epiphyses. This indication is approved under accelerated approval based on an improvement in annualized growth velocity. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). (1)
DOSAGE AND ADMINISTRATION
- Ensure adequate food and fluid intake prior to administration. (2.1)
- Recommended dosage is based on patient's actual body weight. Administer VOXZOGO subcutaneously once daily. (2.2)
- Reconstitute prior to use. Injection volume is based on both patient's weight and concentration of reconstituted VOXZOGO. (2.2)
- Monitor growth and adjust dosage according to actual body weight. Permanently discontinue upon closure of epiphyses. (2.3)
- See full prescribing information for reconstitution, dilution, and administration instructions. (2.4)
DOSAGE FORMS AND STRENGTHS
For injection: 0.4 mg, 0.56 mg, or 1.2 mg of vosoritide as a lyophilized powder in a single-dose vial for reconstitution. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
Risk of Low Blood Pressure: Transient decreases in blood pressure have been reported. Instruct patients to be well-hydrated and have adequate food intake prior to administration of VOXZOGO (5.1)
ADVERSE REACTIONS
Most common adverse reactions (>10%) are injection site erythema, injection site swelling, rash, vomiting, injection site urticaria, arthralgia, decreased blood pressure, and gastroenteritis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact BioMarin Pharmaceutical Inc. at 1-866-906-6100, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Renal Impairment: Not recommended in patients with eGFR < 60 mL/min/1.73 m2. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Instructions Prior to Administration of VOXZOGO
2.2 Recommended Dosage and Administration
2.3 Growth Monitoring
2.4 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Low Blood Pressure
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.6 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Pediatric Patients 5 Years of Age and Older
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VOXZOGO is indicated to increase linear growth in pediatric patients with achondroplasia with open epiphyses. This indication is approved under accelerated approval based on an improvement in annualized growth velocity [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Instructions Prior to Administration of VOXZOGO
To reduce the risk of low blood pressure and its associated signs and symptoms, instruct the caregiver and patient that the patient should [see Warnings and Precautions (5.1)]:
- Have adequate food intake prior to VOXZOGO administration.
- Drink approximately 240 to 300 mL of fluid in the hour prior to VOXZOGO administration.
2.2 Recommended Dosage and Administration
The recommended dosage of VOXZOGO is based on the patient's actual body weight (see Table 1). VOXZOGO is administered by subcutaneous injection once daily [see Dosage and Administration (2.4)].
Inject VOXZOGO at approximately the same time each day, if possible. The volume of VOXZOGO to be administered (injection volume) is based on the patient's actual body weight and the concentration of reconstituted VOXZOGO (0.8 mg/mL or 2 mg/mL) (see Table 1). VOXZOGO must be reconstituted prior to use [see Dosage and Administration (2.4)].
Table 1: Recommended VOXZOGO Daily Dosage and Injection Volume Actual Body Weight* Dose Injection Volume Vial Strength for Reconstitution† 3 kg 0.096 mg 0.12 mL 0.4 mg 4 kg 0.12 mg 0.15 mL 0.4 mg 5 kg 0.16 mg 0.2 mL 0.4 mg 6 to 7 kg 0.2 mg 0.25 mL 0.4 mg 8 to 11 kg 0.24 mg 0.3 mL 0.4 mg 12 to 16 kg 0.28 mg 0.35 mL 0.56 mg 17 to 21 kg 0.32 mg 0.4 mL 0.56 mg 22 to 32 kg 0.4 mg 0.5 mL 0.56 mg 33 to 43 kg 0.5 mg 0.25 mL 1.2 mg 44 to 59 kg 0.6 mg 0.3 mL 1.2 mg 60 to 89 kg 0.7 mg 0.35 mL 1.2 mg ≥ 90 kg 0.8 mg 0.4 mL 1.2 mg 2.3 Growth Monitoring
Monitor and assess patient body weight, growth, and physical development regularly every 3 to 6 months. Adjust the dosage according to the patient's actual body weight [see Dosage and Administration (2.2)].
Permanently discontinue VOXZOGO upon confirmation of no further growth potential, indicated by closure of epiphyses.
2.4 Preparation and Administration
Reconstitute VOXZOGO before administration using the provided diluent syringe containing Sterile Water for Injection, USP (see Reconstitution Instructions below).
Caregivers may inject VOXZOGO subcutaneously after proper training by a healthcare professional on the preparation and administration of VOXZOGO [see Instructions for Use].
Reconstitution Instructions
- Select the correct VOXZOGO vial strength (co-packaged with prefilled syringe with Sterile Water for Injection diluent) based on the patient's actual body weight [see Dosage and Administration (2.2)].
- Remove VOXZOGO vial and prefilled diluent syringe from the refrigerator and allow the vial and prefilled diluent syringe to reach room temperature before reconstituting VOXZOGO.
- Attach the diluent needle provided with ancillary supplies to the diluent prefilled syringe.
- Inject the entire diluent prefilled syringe volume into the vial (see Table 2).
- Gently swirl the diluent in the vial until the white powder is completely dissolved. Do not shake.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Once reconstituted VOXZOGO is a clear, colorless to yellow liquid. The solution should not be used if discolored or cloudy, or if particles are present. The concentration of reconstituted solution is 0.8 mg/mL or 2.0 mg/mL (see Table 2).
- After reconstitution, VOXZOGO can be held in the vial at a room temperature 20°C to 25°C (68°F to 77°F) for a maximum of 3 hours.
- For administration, extract the required dose volume from the vial using the supplied administration syringe [see Dosage and Administration (2.2)].
Table 2: Dilution Requirements for VOXZOGO Prior to Administration Vial Strength Reconstitution Volume Reconstituted Concentration 0.4 mg 0.5 mL 0.8 mg/mL 0.56 mg 0.7 mL 0.8 mg/mL 1.2 mg 0.6 mL 2 mg/mL Discard any unused portion. Do not pool unused portions from the vials. Do not administer more than 1 dose from a vial. Do not mix with other medications.
Instructions for Subcutaneous Administration
See the Instructions for Use document for detailed, illustrated instructions.
- Ensure patients have had adequate food and fluid intake prior to VOXZOGO administration [see Dosage and Administration (2.1)]. Slowly withdraw the dosing volume of the reconstituted VOXZOGO solution from the single-dose vial into a syringe.
- Rotate sites for subcutaneous injections.
- The recommended injection sites for VOXZOGO are: the front middle of the thighs, the lower part of the abdomen at least 2 inches (5 centimeters) away from the navel, top of the buttocks or the back of the upper arms. The same injection area should not be used on two consecutive days. Do not inject VOXZOGO into sites that are red, swollen, or tender.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Low Blood Pressure
Transient decreases in blood pressure were observed in clinical studies of VOXZOGO. Subjects with significant cardiac or vascular disease and patients on anti-hypertensive medicinal products were excluded from participation in VOXZOGO clinical trials. To reduce the risk of a decrease in blood pressure and associated symptoms (dizziness, fatigue and/or nausea), instruct patients to be well hydrated and have adequate food intake prior to administration of VOXZOGO [see Dosage and Administration (2.1) and Adverse Reactions (6.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risk of Low Blood Pressure [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pediatric Patients 5 Years of Age and Older
VOXZOGO was studied in a 52-week, randomized, double-blind, placebo-controlled trial in 121 subjects with achondroplasia (Study 1) [see Clinical Studies (14)].
The subjects' ages ranged from 5.1 to 14.9 years with a mean of 8.7 years. Sixty four (53%) subjects were male and 57 (47%) were female. Overall, 86 (71%) subjects were White, 23 (19%) were Asian, 5 (4%) were Black or African American, and 7 (6%) were classified as "multiple" race. The demographic and baseline characteristics were balanced between treatment groups. The subjects received either VOXZOGO 15 mcg/kg, or placebo administered subcutaneously once daily.
Table 3 shows adverse reactions that occurred in ≥5% of patients treated with VOXZOGO and at a percentage greater than placebo.
Table 3: Adverse Reactions that Occurred in ≥5% of Patients Treated with VOXZOGO and at a Percentage Greater than Placebo in Study 1* Adverse Reaction Placebo
(N=61)
n (%)VOXZOGO
(N=60)
n (%)Abbreviations: N, total number of subjects in the treatment arm; n, number of subjects with the adverse reaction; %, percent of subjects with the adverse reaction. - *
- Includes adverse reactions occurring more frequently in the vosoritide arm and with a risk difference of ≥5% (i.e., difference of >2 subjects) between treatment arms
- †
- Includes the preferred terms: gastroenteritis and gastroenteritis, viral
- ‡
- Includes the preferred terms: dizziness, presyncope, procedural dizziness, vertigo
- §
- Includes the preferred terms: fatigue, lethargy, malaise
Injection site erythema 42 (69%) 45 (75%) Injection site swelling 22 (36%) 37 (62%) Vomiting 12 (20%) 16 (27%) Injection site urticaria 6 (10%) 15 (25%) Arthralgia 4 (7%) 9 (15%) Decreased blood pressure 3 (5%) 8 (13%) Gastroenteritis† 5 (8%) 8 (13%) Diarrhea 2 (3%) 6 (10%) Dizziness‡ 2 (3%) 6 (10%) Ear pain 3 (5%) 6 (10%) Influenza 3 (5%) 6 (10%) Fatigue§ 2 (3%) 5 (8%) Seasonal allergy 1 (2%) 4 (7%) Dry skin 0 3 (5%) Discussion of Selected Adverse Reactions
Decreased blood pressure
Eight (13%) of 60 subjects treated with VOXZOGO had a total of 11 events of transient decrease in blood pressure compared to 3 (5%) of 61 subjects on placebo, identified predominantly during periods of frequent monitoring at clinical visits after dosing over a 52-week treatment period. The median time to onset from injection was 31 (18 to 120) minutes with resolution within 31 (5 to 90) minutes in VOXZOGO-treated subjects. Two out of 60 (3%) VOXZOGO-treated subjects each had one symptomatic episode of decreased blood pressure with vomiting and/or dizziness compared to 0 of 61 (0%) subjects on placebo.
Injection site reactions
Injection site reactions occurred in 51 (85%) subjects receiving VOXZOGO and 50 (82%) subjects receiving placebo over a 52 week period of treatment. Injection site reactions included the preferred terms injection site erythema, injection site reaction, injection site swelling, injection site urticaria, injection site pain, injection site bruising, injection site pruritus, injection site hemorrhage, injection site discoloration, and injection site induration. Over a 52 week period, 51 (85%) of 60 subjects receiving VOXZOGO experienced a total of 6983 events of injection site reactions, while 50 (82%) of 61 subjects receiving placebo experienced a total of 1776 events of injections site reactions, representing 120.4 events per person/year exposure and 29.2 per person/year exposure, respectively. One injection site reaction event could have been associated with one or more injection site reaction symptoms (e.g., injection site swelling, injection site erythema, injection site urticaria, etc.). Two subjects in the VOXZOGO arm discontinued treatment due to adverse reactions of pain and anxiety with injections.
Pediatric Patients <5 Years
The safety of VOXZOGO in pediatric patients <5 years with achondroplasia was evaluated in a 52-week randomized, double blind, placebo-controlled study (Study 2). In this study, 64 patients from 4.4 months to <5 years of age were randomized to receive either a daily vosoritide dose with similar exposure to that characterized to be safe and effective in children with ACH aged ≥5 years old, or placebo. An additional 11 patients received open-label treatment as part of this study. Subjects received 30 mcg/kg while they were <2 years of age. The daily dose for subjects was adjusted to 15 mcg/kg immediately following their 2 year birthday. The most common adverse reactions (>10%) reported in pediatric patients <5 years were injection site reactions (86%) and rash (28%).
The overall safety profile of VOXZOGO in pediatric patients <5 years was similar to that seen in older pediatric patients.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of VOXZOGO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders: hypertrichosis (includes the preferred terms: hair growth abnormal and hypertrichosis).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on vosoritide use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, there was no evidence of embryo-fetal toxicity or congenital malformations when pregnant rats and rabbits were administered vosoritide subcutaneously at doses equivalent to 14-times and 200-times, respectively, the exposure at the maximum recommended human dose (MRHD) (see Data).
The estimated background risk of major birth defects for the indicated population is higher than the general population. The estimated background risk of miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryofetal developmental toxicity study in rats, vosoritide was administered at 90, 270, 540 mcg/kg once daily by subcutaneous injection during the period of major organogenesis from gestation day (GD) 6 – 17. There were no effects on maternal animals or on embryofetal development at the highest dose administered (14-times the exposure at the MRHD).
In an embryofetal developmental toxicity study in rabbits, vosoritide was administered at 45, 135, 240 mcg/kg once daily by subcutaneous injection during the period of major organogenesis (GD 7 – 19). No effects were observed in maternal animals or on embryofetal development at the highest dose administered (200-times the exposure at the MRHD).
In a pre- and postnatal toxicity study in rats, vosoritide was administered at 90, 270, and 540 mcg/kg once daily by subcutaneous injection during the period of major organogenesis and continuing to weaning (GD 6 through postpartum day 20). There were no effects on maternal animals, including maintenance of pregnancy, parturition, or care of offspring, and no effects were noted on offspring growth and development or ability to reproduce at the highest dose (14-times the exposure at the MRHD).
8.2 Lactation
Risk Summary
There is no information regarding the presence of vosoritide in human milk, the effects on the breastfed infant, or the effects on milk production. Vosoritide is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for VOXZOGO and any potential adverse effects on the breastfed child from VOXZOGO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of VOXZOGO have been established in pediatric patients for the improvement in linear growth in patients with achondroplasia with open epiphyses.
Use of VOXZOGO for this indication is supported by evidence from an adequate and well-controlled study in 121 pediatric patients aged 5 to 15 years with achondroplasia, pharmacokinetic data in pediatric patients aged 4.5 months to 15 years, and additional safety data in pediatric patients aged 4.4 months to <5 years [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
-
11 DESCRIPTION
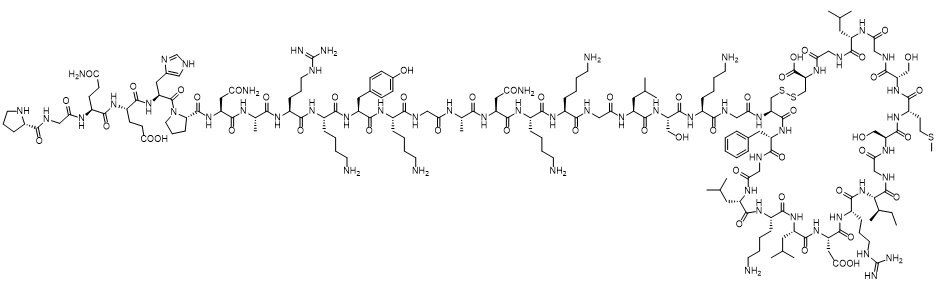
VOXZOGO contains vosoritide, a human C type natriuretic peptide (CNP) analog. Vosoritide is a 39 amino acid peptide. Its amino acid sequence includes the 37 C terminal amino acids of the human CNP53 sequence plus Pro Gly on the N terminus to convey resistance to neutral endopeptidase (NEP) degradation. Vosoritide is manufactured from Escherichia coli using recombinant DNA technology. Vosoritide has a chemical formula of C176H290N56O51S3 with a molecular weight of 4.1 kDa.
Vosoritide has the structural formula shown in Figure 1.
Figure 1
VOXZOGO (vosoritide) for injection, is a sterile, preservative-free white-to-yellow lyophilized powder, for subcutaneous administration after reconstitution with Sterile Water for Injection, USP.
VOXZOGO is provided as a single-dose vial containing 0.4 mg, 0.56 mg, or 1.2 mg of vosoritide per vial. A pre-filled syringe containing Sterile Water for Injection, USP for use as a diluent is also provided. The contents of each single dose vial are summarized by strength in Table 4. The product contains no preservative.
Table 4: Contents of VOXZOGO Strength Inactive Ingredients per Vial Trehalose dihydrate and D-Mannitol are used as isotonic agent. Citric acid monohydrate and sodium citrate dihydrate are used as buffering agent. VOXZOGO 0.4 mg Citric acid monohydrate (0.14 mg), mannitol (7.5 mg), methionine (0.36 mg), polysorbate 80 (0.025 mg), sodium citrate dihydrate (0.54 mg) and trehalose dihydrate (29.01 mg). After reconstitution with 0.5 mL Sterile Water for Injection USP, the resulting concentration is 0.4 mg/0.5 mL of vosoritide and the nominal deliverable volume is 0.4 mL. VOXZOGO 0.56 mg Citric acid monohydrate (0.20 mg), mannitol (10.50 mg), methionine (0.51 mg), polysorbate 80 (0.035 mg), sodium citrate dihydrate (0.76 mg) and trehalose dihydrate (40.61 mg). After reconstitution with 0.7 mL Sterile Water for Injection USP, the resulting concentration is 0.56 mg/0.7 mL of vosoritide and the nominal deliverable volume is 0.6 mL. VOXZOGO 1.2 mg Citric acid monohydrate (0.17 mg), mannitol (9 mg), methionine (0.44 mg), polysorbate 80 (0.030 mg), sodium citrate dihydrate (0.65 mg) and trehalose dihydrate (34.81 mg). After reconstitution with 0.6 mL Sterile Water for Injection USP, the resulting concentration is 1.2 mg/0.6 mL of vosoritide and the nominal deliverable volume is 0.5 mL. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In patients with achondroplasia, endochondral bone growth is negatively regulated due to a gain of function mutation in fibroblast growth factor receptor 3 (FGFR3). Binding of vosoritide to natriuretic peptide receptor-B (NPR-B) antagonizes FGFR3 downstream signaling by inhibiting the extracellular signal-regulated kinases 1 and 2 (ERK1/2) in the mitogen-activated protein kinase (MAPK) pathway at the level of rapidly accelerating fibrosarcoma serine/threonine protein kinase (RAF-1). As a result, vosoritide, like CNP, acts as a positive regulator of endochondral bone growth as it promotes chondrocyte proliferation and differentiation.
In animal models with open growth plates, vosoritide administration resulted in the promotion of chondrocyte proliferation and differentiation that led to a widening of the growth plate and subsequent increase in skeletal growth. In the mouse models of FGFR3-related chondrodysplasia, a partial or complete normalization of the dwarfism phenotype was observed.
12.2 Pharmacodynamics
NPR-B Binding Activity Biomarker and Bone Metabolism Biomarker
An increase in urinary cyclic guanosine monophosphate (cGMP) concentrations from pre-dose baseline were observed within the first four hours post-dose, with a maximum level at 2 hours post-dose, after VOXZOGO administration to pediatric patients with achondroplasia.
Daily administration of VOXZOGO also led to the increase from baseline in serum collagen type X marker (CXM), an endochondral ossification biomarker and remains elevated beyond 24 months. In subjects aged 5 - 14 years old at screening, exposure-response analyses showed that vosoritide activity measured by urinary cGMP was near saturation at the dose of 15 mcg/kg once daily, while maximal increase in growth plate activity indicated by CXM was achieved at this dose.
12.3 Pharmacokinetics
The area under the concentration-time curve (AUC) and peak concentration (Cmax) of vosoritide increased greater than proportionally following subcutaneous administration to pediatric subjects with achondroplasia in the dose range of 7.5 to 30.0 mcg/kg. The pharmacokinetics of vosoritide were evaluated in 58 subjects aged 5 to 13 years with achondroplasia who received subcutaneous injections of vosoritide 15 mcg/kg once daily for 52 weeks. The mean (± SD) Cmax and area under the concentration-time curve from time zero to the last measurable concentration (AUC0-t) observed across 52 weeks of treatment ranged from 4.71 (± 2.32) to 7.18 (± 9.65) ng/mL, and 161 (± 98.1) to 290 (± 235) ng-min/mL, respectively. No drug accumulation was observed following 15 mcg/kg once daily dosing. The exposure of vosoritide increased with the duration of treatment. The mean AUC0-t at week 52 increased approximately 20% compared to that at day 1.
Absorption
Absolute bioavailability for vosoritide following subcutaneous injection was not determined. Vosoritide was absorbed with a median Tmax of 15 minutes after dosing.
Distribution
The mean (± SD) apparent volume of distribution of vosoritide across 52 weeks of subcutaneous administration of VOXZOGO 15 mcg/kg once daily ranged from 2880 (± 2450) to 3020 (± 1980) mL/kg.
Elimination
The mean (± SD) apparent clearance of vosoritide across 52 weeks of subcutaneous administration of VOXZOGO 15 mcg/kg once daily ranged from 79.4 (± 53.0) to 104 (± 98.8) mL/min/kg. The mean (± SD) half-life ranged from 21.0 (± 4.7) to 27.9 (± 9.9) minutes.
Specific Populations
No clinically significant differences in the vosoritide pharmacokinetics were observed based on age (0.4 to 15 years), sex or race. The effect of hepatic or renal impairment on the pharmacokinetics of vosoritide is unknown.
Body weight
Population pharmacokinetic analyses indicated that body weight is a significant covariate for vosoritide clearance and volume of distribution. The apparent clearance and volume of distribution of vosoritide increased with increasing body weight in patients with achondroplasia (5 to 74.5 kg).
Drug Interaction Studies
In vitro assessment of drug-drug interactions
In vitro studies showed that vosoritide, at therapeutic concentrations, does not inhibit or induce Cytochrome P450 enzymes. Based on in vitro studies, vosoritide is considered unlikely to inhibit the human drug uptake or efflux transporters such as OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, MATE1, MATE2-K, BCRP, P-gp, and BSEP at clinically relevant concentrations and therefore, no effect of vosoritide is anticipated on concomitantly administered drugs that are substrates of these transporters.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of vosoritide.
Of 131 subjects aged 5 years and older who were treated with VOXZOGO 15 mcg/kg/day and evaluable for the presence of anti-drug antibodies (ADA) for up to 240 weeks, ADA were detected in 35% (46/131). The earliest time to ADA development was day 85. All ADA-positive subjects tested negative for anti-vosoritide neutralizing antibodies. There was no correlation between the number, duration, or severity of hypersensitivity adverse reactions or injection site reactions and ADA positivity or mean ADA titer. There was no association between ADA positivity or mean ADA titer and change from baseline in annual growth velocity (AGV) or height Z-score at month 12. There was no impact of serum ADA detected on the plasma PK measurements of vosoritide.
In subjects under 5 years of age, 33% (20/61) of vosoritide-treated subjects tested positive for ADA and all placebo-treated subjects tested negative for ADA for up to 44 months. The earliest time to ADA development was week 26. All of the ADA-positive subjects tested negative for neutralizing antibodies at all time points. There was no impact of ADA development on safety, efficacy or PK of vosoritide up to Month 12.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Pediatric Patients 5 Years of Age and Older
The safety and effectiveness of VOXZOGO in patients with achondroplasia were assessed in one 52-week, multi-center, randomized, double-blind, placebo-controlled, phase 3 study - Study 1 (NCT03197766).
Study 1 was conducted in 121 subjects with genetically-confirmed achondroplasia, who were randomized to either VOXZOGO (N=60) or placebo (N=61). The dosage of VOXZOGO was 15 mcg/kg administered subcutaneously once daily. Baseline standing height, weight Z-score, body mass index (BMI) Z-score, and upper to lower body ratio were collected for at least 6 months prior to randomization. Subjects with limb-lengthening surgery in the prior 18 months or who planned to have limb-lengthening surgery during the study period were excluded. The study included a 52-week placebo-controlled treatment phase followed by an open-label treatment extension study period in which all subjects received VOXZOGO. The primary efficacy endpoint was the change from baseline in annualized growth velocity (AGV) at Week 52 compared with placebo.
The subjects' ages ranged from 5.1 to 14.9 years with a mean of 8.7 years. Sixty four (53%) subjects were male and 57 (47%) were female. Overall, 86 (71%) subjects were White, 23 (19%) were Asian, 5 (4%) were Black or African American, and 7 (6%) were classified as "multiple" race. The subjects had a mean baseline height standard deviation score (SDS) of -5.13.
Treatment with VOXZOGO for 52 weeks resulted in a treatment difference in the change from baseline in AGV of 1.57 cm/year after 52 weeks of treatment (Table 5).
Table 5: Annualized Growth Velocity (cm/year) at Week 52 in Subjects 5 Years of Age and Older with Achondroplasia - Study 1 Placebo
(N=61*)VOXZOGO 15 mcg/kg Daily
(N=60*)Abbreviations: AGV, annualized growth velocity; 95% CI, 95% confidence interval; LS, least-square; SD, standard deviation - *
- All randomized subjects. Two patients in the VOXZOGO group discontinued from the study before Week 52. The values for these 2 patients were imputed assuming baseline growth rate for the period with missing data.
- †
- Baseline AGV was based on standing height at least 6 months prior to enrollment into the study.
- ‡
- LS means were estimated from the ANCOVA (analysis of covariance) model, which included treatment, stratum defined by sex and Tanner stage, baseline age, baseline AGV and baseline height Z-score.
- §
- 2-sided p-value <0.0001 for superiority.
Baseline mean (SD)† 4.06 (1.20) 4.26 (1.53) Change from baseline‡ -0.17 1.40 Difference in change of VOXZOGO – Placebo‡
(95% CI)1.57
(1.22, 1.93)§The improvement in AGV in favor of VOXZOGO was consistent across all predefined subgroups analyzed including sex, age group, Tanner stage, baseline height Z-score, and baseline AGV.
Height Standard Deviation Score (SDS)
The LS mean change from baseline to Week 52 in height SDS was -0.02 in the placebo group and 0.26 in the VOXZOGO group. The difference in LS mean change from baseline was 0.28 (95% CI 0.17, 0.39; p<0.0001) in favor of VOXZOGO. The LS mean change from baseline to Week 52 in upper to lower body segment ratio was -0.02 in the placebo group and -0.03 in the VOXZOGO group. The difference in LS mean change from baseline was -0.01 (95% CI -0.05, 0.02; p=0.5).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
VOXZOGO for injection is a white to yellow lyophilized powder for reconstitution and is provided as a co-pack which includes ten:
- Sterile, single-dose 2 mL glass vials containing VOXZOGO
- Diluent (Sterile Water for Injection, USP) in a single-dose prefilled syringe
- Diluent transfer needles (23 gauge)
- Single-dose administration syringes (30 gauge) both with needle retraction safety devices
Strength (mg) Diluent (mL) Co-pack NDC Number Flip Cap Color 0.4 0.5 NDC 68135-082-36 White 0.56 0.7 NDC 68135-119-66 Magenta 1.2 0.6 NDC 68135-181-93 Grey The following items to be obtained separately; alcohol aseptic wipes, gauze, bandages and sharps container.
Storage
Refrigerate VOXZOGO vials and prefilled diluent syringes at 2°C to 8°C (36°F to 46°F). Do not freeze.
VOXZOGO can be stored at room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) for 90 days. Do not return VOXZOGO to the refrigerator once stored at room temperature.
After reconstitution, VOXZOGO can be held in the vial at room temperature 20°C to 25°C (68°F to 77°F) for a maximum of 3 hours [see Dosage and Administration (2.4)].
Record the starting date of room-temperature storage clearly on the unopened product carton.
Do not use beyond expiration date on the label.
Store in the original package to protect from light.
Handling
Reconstituted VOXZOGO must be administered within 3 hours of reconstitution [see Dosage and Administration (2.4)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Preparation and Administration
Instruct caregivers on proper preparation and administration of VOXZOGO. Ensure caregivers have demonstrated the ability to perform a subcutaneous injection [see Dosage and Administration (2.4)].
Instruct caregivers in the technique of proper syringe and needle disposal, and advise them not to reuse these items. Instruct caregivers to dispose needles and syringes in a puncture-resistant container.
Risk of Low Blood Pressure
Inform caregivers and patients that VOXZOGO may lower blood pressure after administration. Instruct caregivers and patients that prior to VOXZOGO administration, the patient should have adequate food intake and within the hour prior to administration, the patient should drink approximately 8-10 ounces (240-300 mL) of fluid [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
VOXZOGO (vox zoeʹ goe)
(vosoritide)
for injection, for subcutaneous useThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 10/2023 What is VOXZOGO?
VOXZOGO is a prescription medicine used to increase linear growth in children with achondroplasia with open bone growth plates (epiphyses).Before you give your child VOXZOGO, tell your child's healthcare provider about all your child's medical conditions, including if they: - have kidney problems.
- are pregnant or plan to become pregnant. It is not known if VOXZOGO will harm your child's unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if VOXZOGO passes into your child's breast milk. Talk to your child's healthcare provider about the best way to feed your child's baby if your child takes VOXZOGO.
Know the medicines your child takes. Keep a list of them to show your child's healthcare provider and pharmacist when your child gets a new medicine.How should I give VOXZOGO? - See the detailed Instructions for Use that comes with this Patient Information leaflet for instructions about the right way to store, prepare, and give VOXZOGO injections at home.
- VOXZOGO is given as an injection under the skin (subcutaneous or SC). Inject VOXZOGO 1 time every day, at about the same time each day.
- If your child's healthcare provider decides a caregiver can give the injections of VOXZOGO at home, your child's caregiver should receive training on the right way to prepare and inject VOXZOGO. Do not try to inject VOXZOGO until you have been shown the right way by your child's healthcare provider or nurse.
- Your child's healthcare provider will tell you how often you should give VOXZOGO. If your child misses a dose of VOXZOGO, it can be given within 12 hours of the scheduled time of injection. If more than 12 hours have passed, do not give the missed dose. Give the next daily dose according to your child's usual schedule.
- Your child should eat a meal and drink about 8 to 10 ounces of fluid within 1 hour before injection.
- In case you are not sure when to inject VOXZOGO, call your child's healthcare provider or pharmacist. Do not give VOXZOGO more often than as directed by your child's healthcare provider.
- Your child's dose of VOXZOGO depends on his or her body weight. Your child's healthcare provider will tell you which strength of VOXZOGO to use and how much to give your child.
- Your child's healthcare provider will monitor your child's growth and instruct you on when your child should stop VOXZOGO if they determine that your child is no longer able to grow. Stop giving VOXZOGO to your child if instructed by your child's healthcare provider.
What are the possible side effects of VOXZOGO?
VOXZOGO may cause serious side effects, including:- risk of low blood pressure. VOXZOGO may temporarily lower blood pressure in some people. To help reduce the risk of low blood pressure and its symptoms (dizziness, feeling tired, or nausea), your child should eat a meal and drink about 8 to 10 ounces of fluid within 1 hour before receiving VOXZOGO.
- injection site reactions (redness, itching, swelling, bruising, rash, hives, pain)
- high levels of blood alkaline phosphatase (shown in blood tests)
- vomiting
- joint pain
- decreased blood pressure
- stomach ache
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store VOXZOGO? - Store the VOXZOGO vial and prefilled diluent syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- You may store VOXZOGO (before mixing) at room temperature between 68°F to 77°F (20°C to 25°C) for 90 days.
- Record the date you started storing VOXZOGO at room temperature on the carton to keep track of the 90 days.
- Do not return VOXZOGO to the refrigerator after it has been stored at room temperature. Throw VOXZOGO away if unused within 90 days of storing at room temperature.
- Do not use VOXZOGO past the expiration date.
- Do not freeze VOXZOGO.
- Store VOXZOGO out of direct sunlight.
General information about the safe and effective use of VOXZOGO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VOXZOGO for a condition for which it was not prescribed.
Do not give VOXZOGO to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about VOXZOGO that is written for health professionals.What are the ingredients in VOXZOGO?
Active ingredient: vosoritide
Inactive ingredients: trehalose dihydrate, mannitol, sodium citrate dihydrate, methionine, citric acid monohydrate, and polysorbate 80
BioMarin Pharmaceutical Inc.
Novato, CA 94949
© BioMarin Pharmaceutical Inc. All rights reserved.
VOXZOGO is a registered trademark of BioMarin Pharmaceutical Inc.
For more information, go to www.VOXZOGO.com or call 1-877-695-8826. -
INSTRUCTIONS FOR USEVOXZOGO™ [Vox zoeʹ goe](vosoritide)for injection, for subcutaneous useSingle-Use
This Instructions for Use contains information for caregivers on how to inject VOXZOGO.
Read this Instructions for Use before you start using VOXZOGO and each time you get a refill. There may be new information. This information does not take the place of talking to your child's healthcare provider about your child's medical condition and their treatment. Before you use VOXZOGO for the first time, make sure your child's healthcare provider shows you the right way to use it. Contact your child's healthcare provider if you or your child have any questions.
Important Information You Need to Know Before Injecting VOXZOGO
- Wash your hands with soap and water.
- Do not drop VOXZOGO or put opened items down on surfaces that are not clean.
- VOXZOGO is available in more than 1 strength. Make sure the strength matches your prescription strength. Do not open packaging until ready to use.
- Take the VOXZOGO vial and prefilled diluent syringe out of the refrigerator and allow them to reach room temperature before mixing.
- Inspect the vial and supplies for any signs of damage or contamination. Do not use if damaged or contaminated.
- Check the expiration date. The expiration date can be found on the carton, vial and prefilled diluent syringe. Do not use if expired.
- Your child should eat a meal and drink a glass (about 8 to 10 ounces) of fluid (such as water, milk, or juice) within 1 hour before injection.
- VOXZOGO should be given at about the same time every day.
- Do not mix VOXZOGO with other medicines.
- After mixing the VOXZOGO, use it right away. Do not use the mixed VOXZOGO if it has been sitting at room temperature for more than 3 hours. Throw it away (dispose of) in a sharps container. See step 18 and "How to Throw Away (Dispose of) VOXZOGO" for more information.
- Do not reuse any of the supplies. After the injection, throw away (dispose of) the used vial even if there is VOXZOGO remaining. See step 18 and "How to Throw Away (Dispose of) VOXZOGO" for more information.
How to Store VOXZOGO
- Store the VOXZOGO vial and prefilled diluent syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- You may store VOXZOGO (before mixing) at room temperature between 68°F to 77°F (20°C to 25°C) for 90 days. Record the date you started storing VOXZOGO at room temperature on the carton to keep track of the 90 days. Do not return VOXZOGO to the refrigerator after it has been stored at room temperature. Throw VOXZOGO away if unused within 90 days of storing at room temperature.
- Do not freeze VOXZOGO.
- Store VOXZOGO out of direct sunlight.
Keep VOXZOGO and all other medicines out of the reach of children.
Supplies Needed to Inject VOXZOGO
Gather all of these supplies on a clean, flat surface before injecting.
Items supplied Items not supplied
If you do not have these items, ask your pharmacist.VOXZOGO
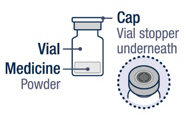

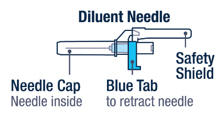

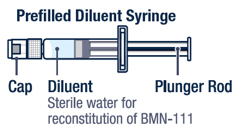
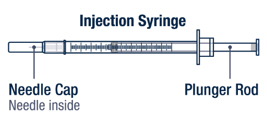
Preparing VOXZOGO for Injection
▶ Step 1
On a clean flat surface, flip off the vial cap and wipe the top with an alcohol pad.▶ Step 2
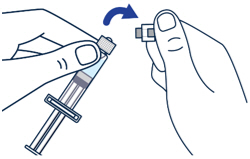
Gently bend to snap off the cap from the prefilled diluent syringe.▶ Step 3
Twist the diluent needle onto the prefilled diluent syringe until you can no longer twist it. Do not use the prefilled diluent syringe to give the injection.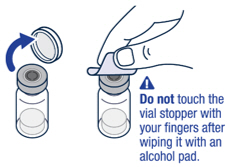
▶ Step 4
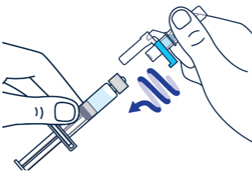
Pull off the needle cap and insert the needle through the middle of the vial stopper. Slowly push the plunger rod down to inject all of the liquid.▶ Step 5
Remove the needle from the vial, then press the blue tab for the needle to pull back (retract). Throw away the needle and syringe in a sharps container. See step 18 and "How to Throw Away (Dispose of) VOXZOGO." Do not use the prefilled diluent syringe to give the injection.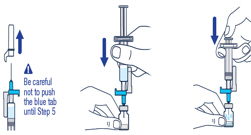
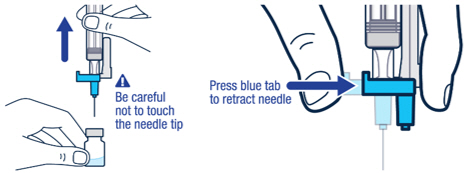
▶ Step 6
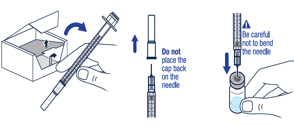
Gently swirl the vial until the powder has completely dissolved and the solution is clear. Do not shake.▶ Step 7
Take the injection syringe out of the carton. Pull off the needle cap from the injection syringe and insert the needle straight through the middle of the vial stopper. Be careful not to bend the needle.▶ Step 8
Carefully hold the vial and syringe and turn the vial upside down with the needle still inserted. The vial should be on top. Be careful not to bend the needle.
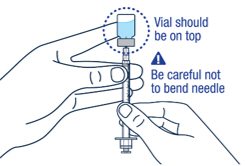
▶ Step 9
Keep the needle tip in the medicine and slowly pull the plunger rod back to draw up the prescribed dose in the syringe. Check the prescription label for how much to draw up.▶ Step 10
Remove large air bubbles in the syringe by gently tapping the syringe. Then push the bubbles back into the vial.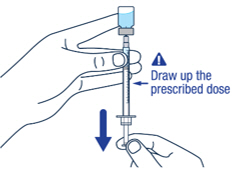
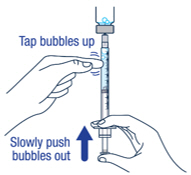
▶ Step 11
Repeat steps 9 and 10 until you have the correct prescribed dose in the syringe and no large bubbles.▶ Step 12
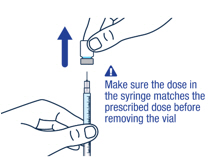
Make sure you have the prescribed dose in the syringe, then remove the vial and prepare to give the dose.
Select and Prepare Injection Site
▶ Step 13 ▶ Step 14 VOXZOGO should be injected into the fatty layer under the skin (subcutaneous) only.
Do not inject into the same site 2 times in a row.Wipe the injection site with an alcohol pad and let the skin air dry. 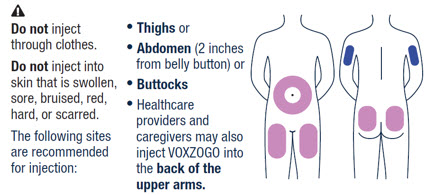
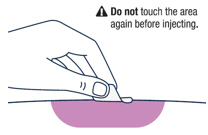
Giving VOXZOGO Injection
▶ Step 15 ▶ Step 16 After wiping the site with an alcohol pad, pinch the skin up around the selected injection site. Quickly insert the needle all the way into the skin at a 45-degree angle. 
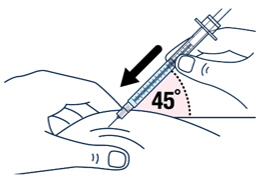
▶ Step 17
Release the pinch and slowly push the plunger rod all the way down.

Continue pressing the plunger rod until the needle retracts into the syringe. ▶ Step 18
Throw away the used vial, syringes and needles in a sharps container. See "How to Throw Away (Dispose of) VOXZOGO" for more information.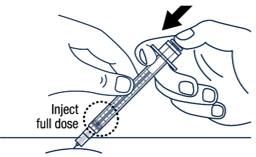
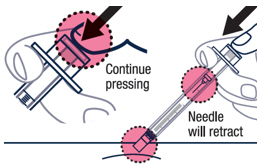
How to Throw Away (Dispose of) VOXZOGO
Put your used or expired vials, needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the vials, loose needles and syringes in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that:
- is made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid without sharps being able to come out,
- is upright and stable during use,
- is leak-resistant, and
- is properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be local or state laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
After the Injection
- Inspect the injection site. If a small amount of bleeding occurs from the injection site, gently press a gauze pad on it for a few seconds or apply a bandage. Do not rub the injection site.
- Monitor for signs of low blood pressure, such as dizziness, tiredness, and nausea. If your child experiences these symptoms you should call your child's healthcare provider, then get your child to lay back with legs raised.
For Help or More Information
- Call your healthcare provider
- Call BioMarin at 1-800-123-4567
- Visit www.VOXZOGO.com
Manufactured for:
BioMarin Pharmaceutical Inc.,
Novato, CA 94949REP-5233-C10
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: November 2021 -
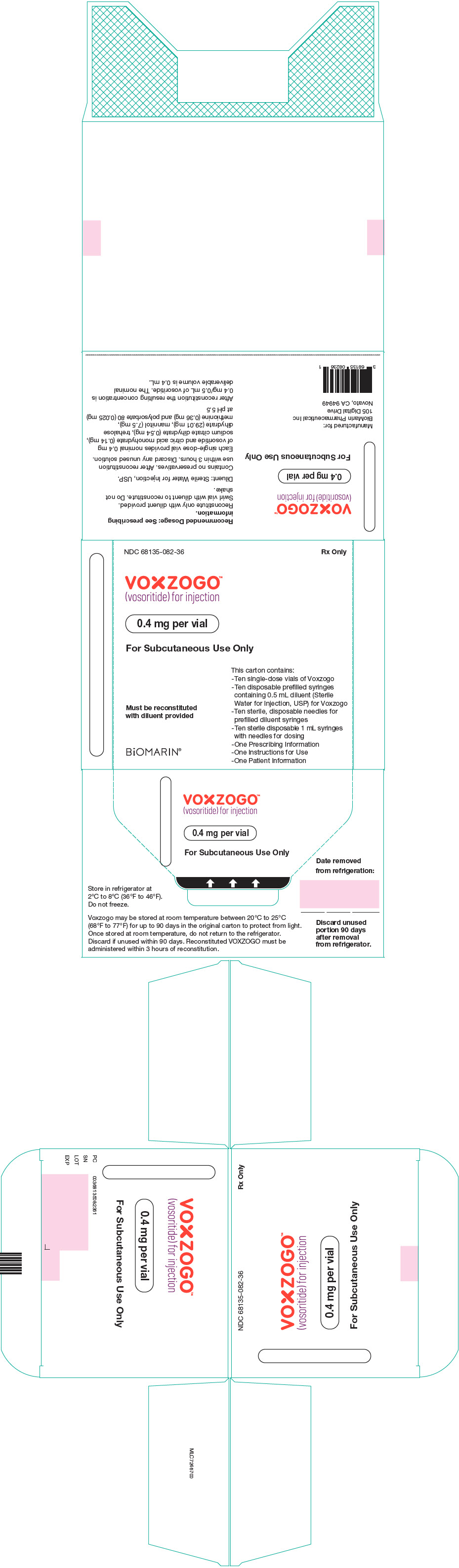
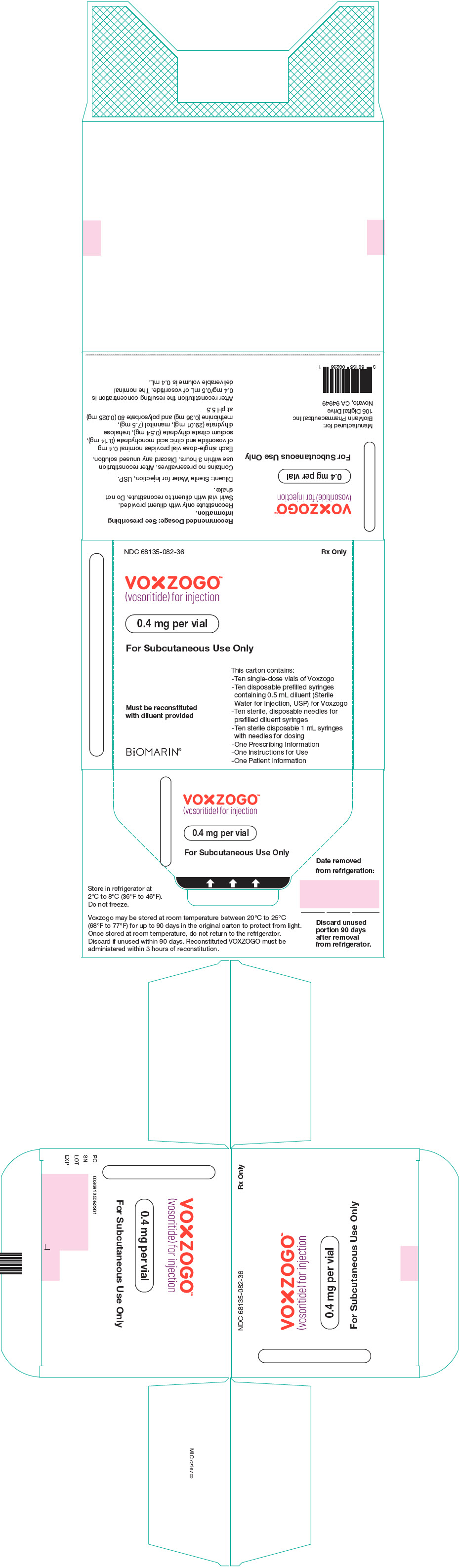
PRINCIPAL DISPLAY PANEL - Kit Carton - 68135-082
NDC 68135-082-36
Rx Only
VOXZOGO™
(vosoritide) for injection0.4 mg per vial
For Subcutaneous Use Only
This carton contains:
- Ten single-dose vials of Voxzogo
- Ten disposable prefilled syringes
containing 0.5 mL diluent (Sterile
Water for Injection, USP) for Voxzogo
- Ten sterile, disposable needles for
prefilled diluent syringes
- Ten sterile disposable 1 mL syringes
with needles for dosing
- One Prescribing Information
- One Instructions for Use
- One Patient InformationMust be reconstituted
with diluent providedBiOMARIN®
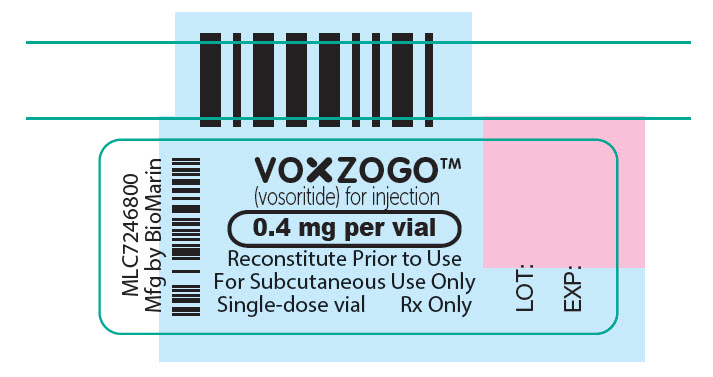
- PRINCIPAL DISPLAY PANEL - 0.4 mg Vial Label
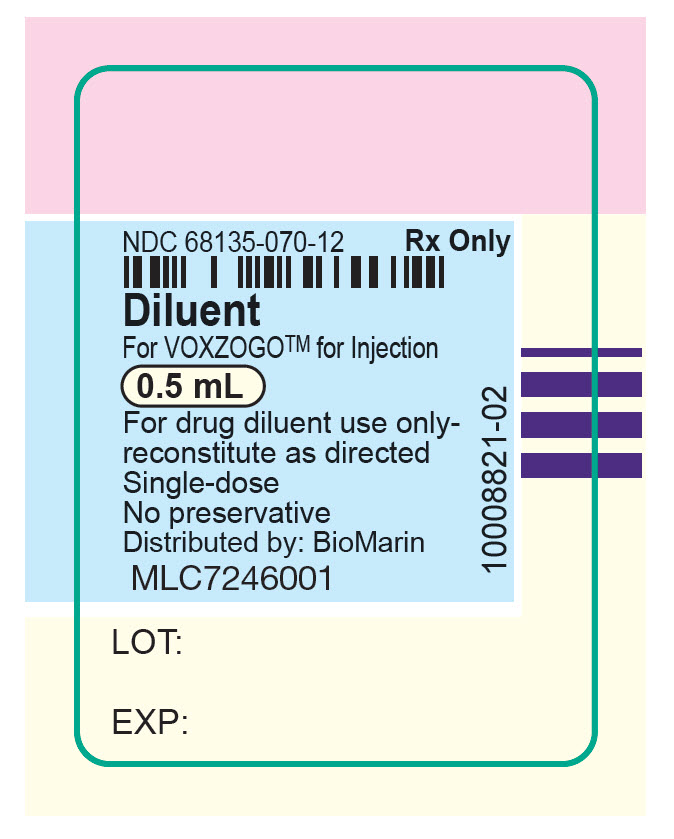
- PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label
-
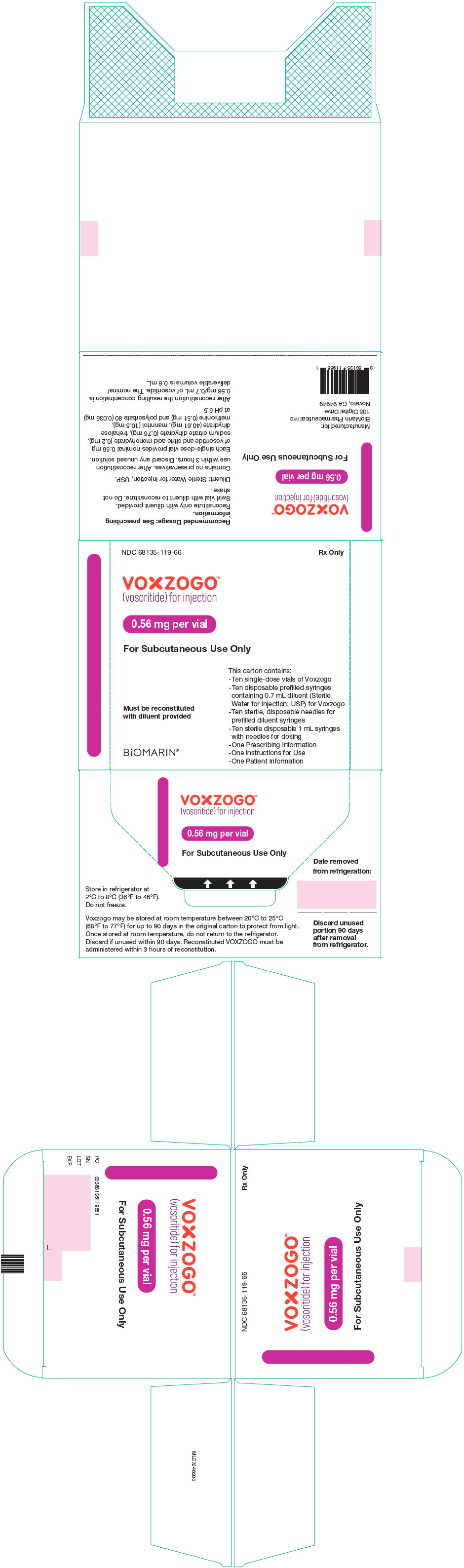
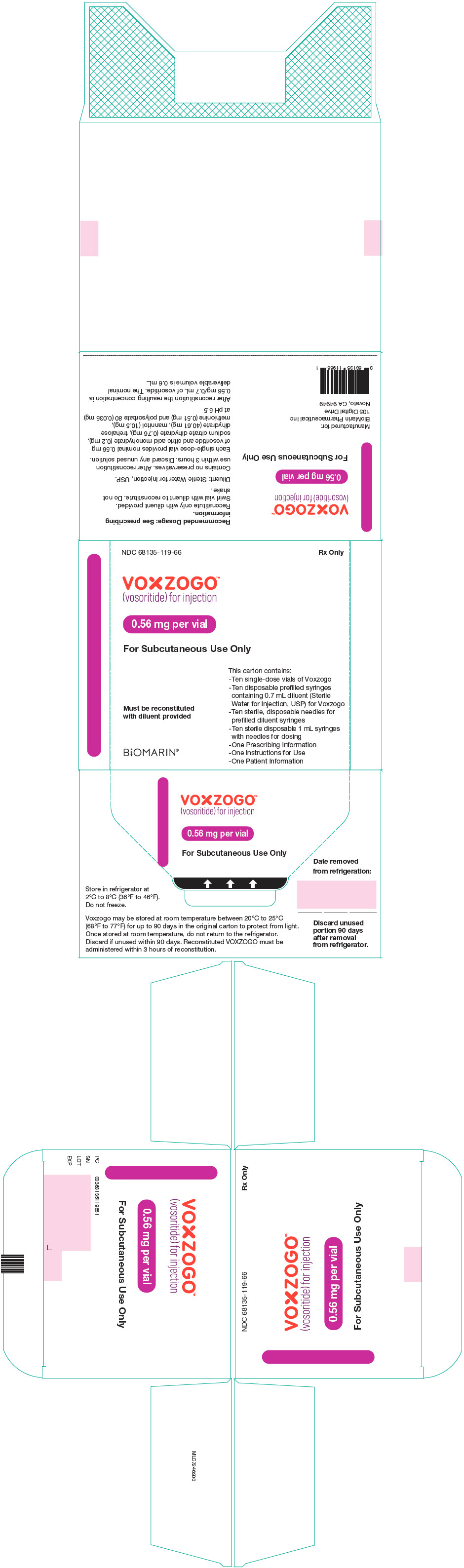
PRINCIPAL DISPLAY PANEL - Kit Carton - 68135-119
NDC 68135-119-66
Rx Only
VOXZOGO™
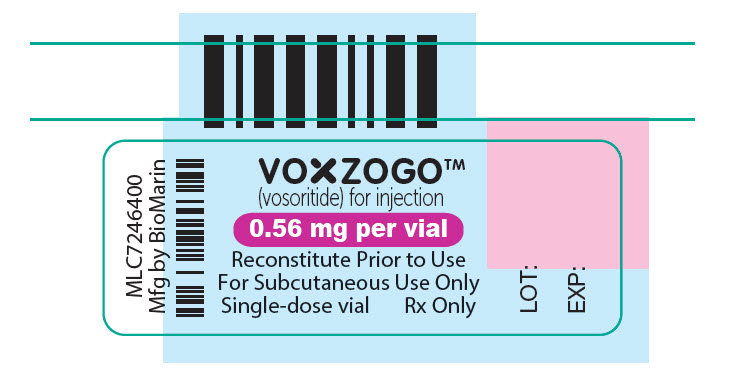
(vosoritide) for injection0.56 mg per vial
For Subcutaneous Use Only
This carton contains:
- Ten single-dose vials of Voxzogo
- Ten disposable prefilled syringes
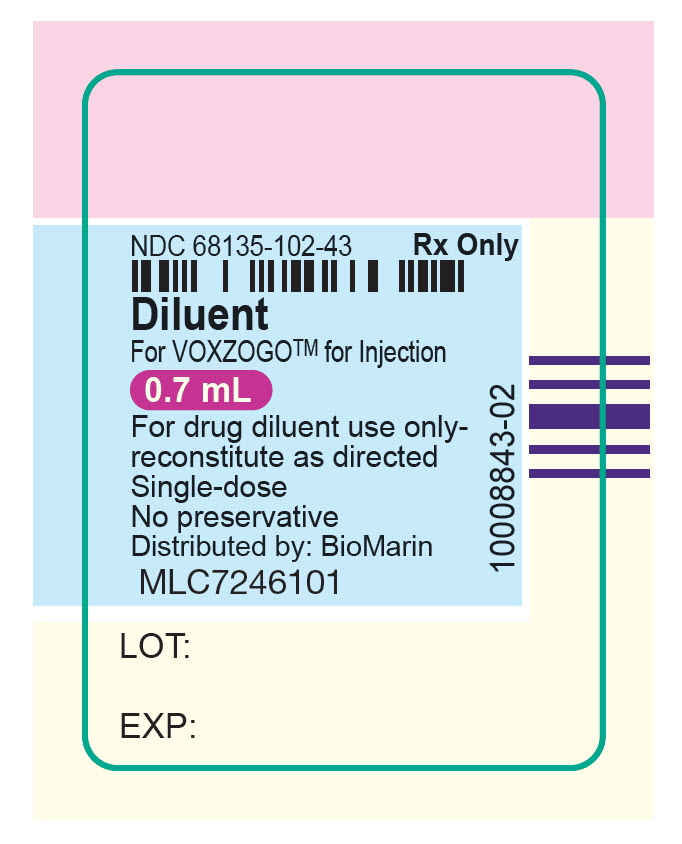
containing 0.7 mL diluent (Sterile
Water for Injection, USP) for Voxzogo
- Ten sterile, disposable needles for
prefilled diluent syringes
- Ten sterile disposable 1 mL syringes
with needles for dosing
- One Prescribing Information
- One Instructions for Use
- One Patient InformationMust be reconstituted
with diluent providedBiOMARIN®
- PRINCIPAL DISPLAY PANEL - 0.56 mg Vial Label
- PRINCIPAL DISPLAY PANEL - 0.7 mL Syringe Label
-
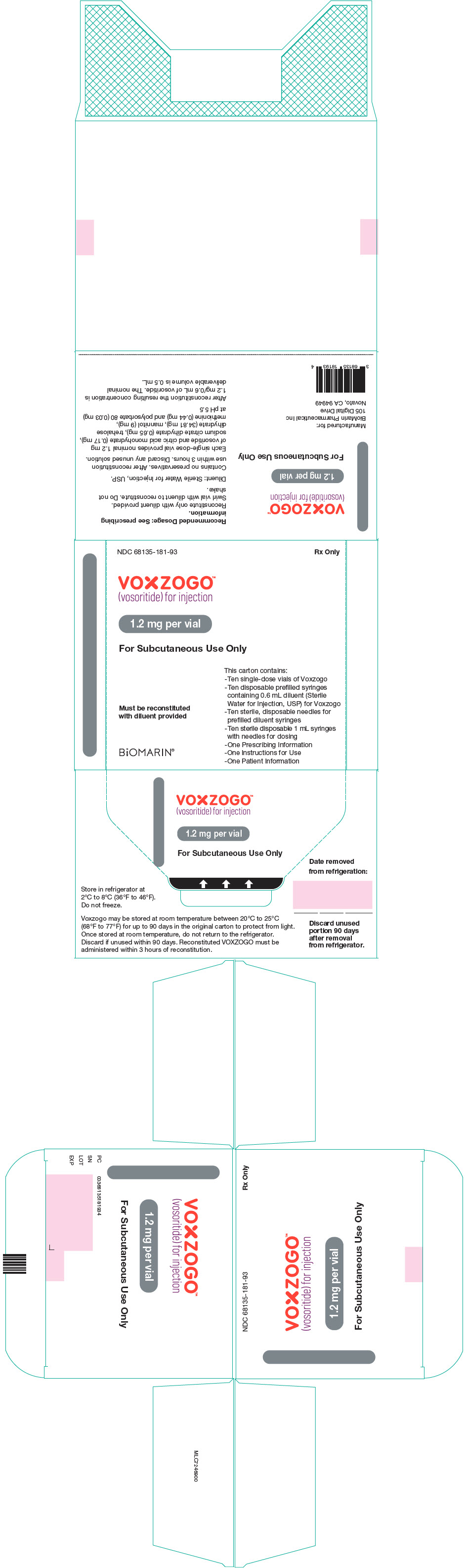
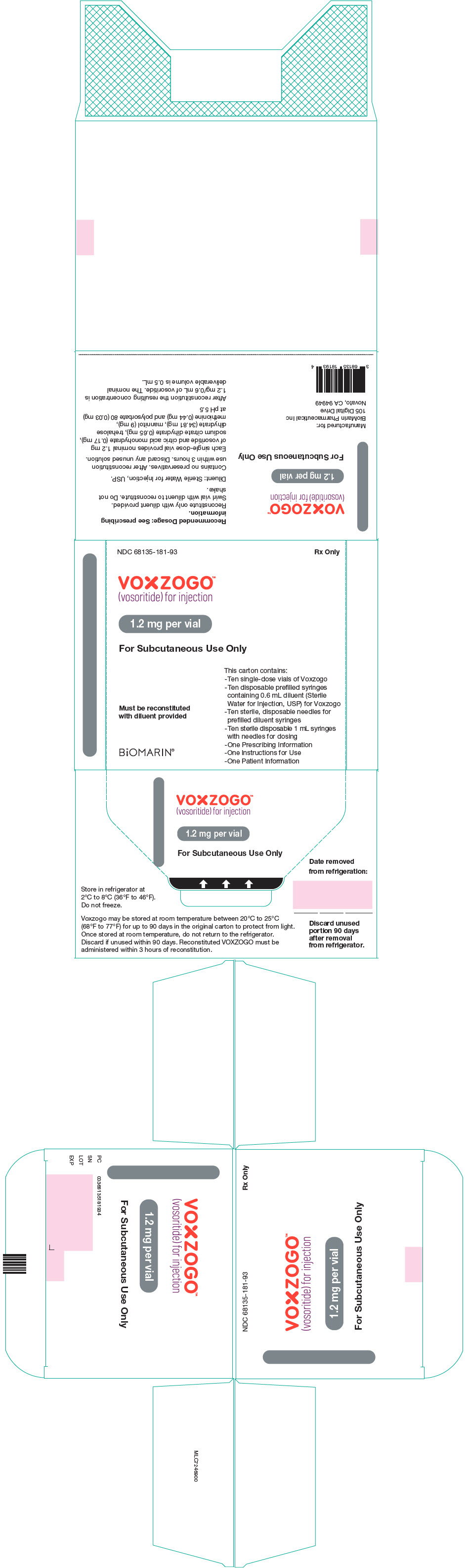
PRINCIPAL DISPLAY PANEL - Kit Carton - 68135-181
NDC 68135-181-93
Rx Only
VOXZOGO™
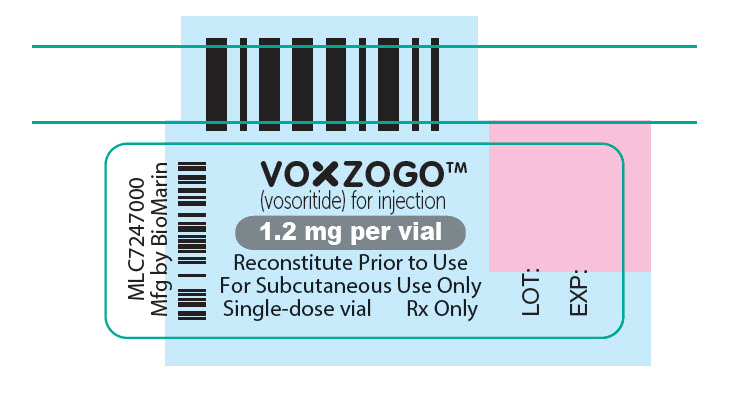
(vosoritide) for injection1.2 mg per vial
For Subcutaneous Use Only
This carton contains:
- Ten single-dose vials of Voxzogo
- Ten disposable prefilled syringes
containing 0.6 mL diluent (Sterile
Water for Injection, USP) for Voxzogo
- Ten sterile, disposable needles for
prefilled diluent syringes
- Ten sterile disposable 1 mL syringes
with needles for dosing
- One Prescribing Information
- One Instructions for Use
- One Patient InformationMust be reconstituted
with diluent providedBiOMARIN®
- PRINCIPAL DISPLAY PANEL - 1.2 mg Vial Label
- PRINCIPAL DISPLAY PANEL - 0.6 mL Syringe Label
-
INGREDIENTS AND APPEARANCE
VOXZOGO 0.4MG
vosoritide kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68135-082 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-082-36 1 in 1 CARTON 11/19/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 VIAL, SINGLE-DOSE 5 mL Part 2 1 CARTON 10 Part 1 of 2 VOXZOGO 0.4MG VIAL
vosoritide injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:68135-061 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VOSORITIDE (UNII: 7SE5582Q2P) (VOSORITIDE - UNII:7SE5582Q2P) VOSORITIDE 0.4 mg in 0.5 mL Product Characteristics Color WHITE (white to yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-061-00 10 in 1 CARTON 1 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214938 11/19/2021 Part 2 of 2 0.5ML DILUENT FOR 0.4MG VOXOGO KIT
water injection, solutionProduct Information Item Code (Source) NDC:68135-070 Route of Administration SUBCUTANEOUS Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-070-12 10 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214938 11/19/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214938 11/19/2021 VOXZOGO 0.56MG
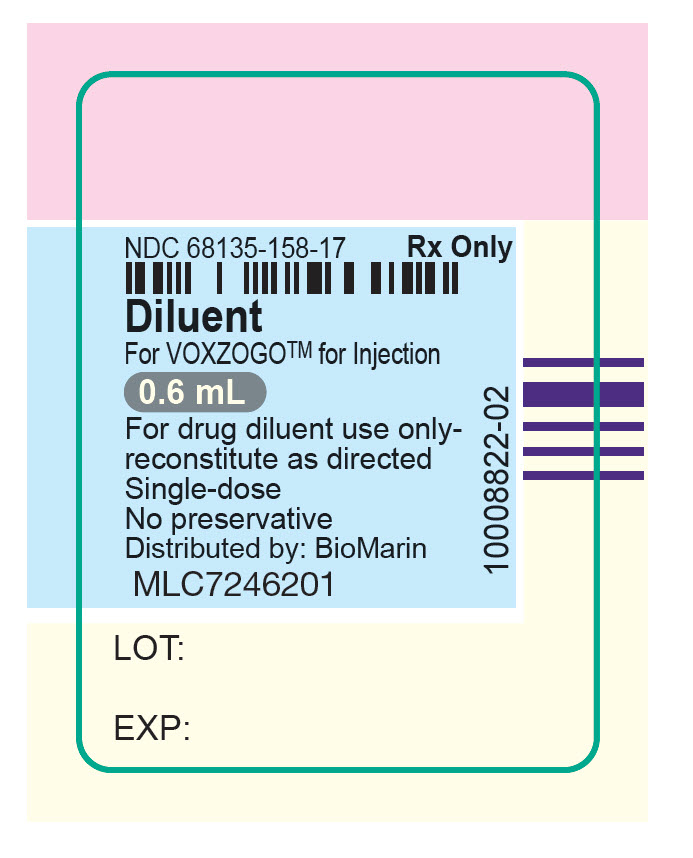
vosoritide kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68135-119 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-119-66 1 in 1 CARTON 11/19/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 VIAL, SINGLE-DOSE 7 mL Part 2 1 CARTON 10 Part 1 of 2 VOXZOGO 0.56MG VIAL
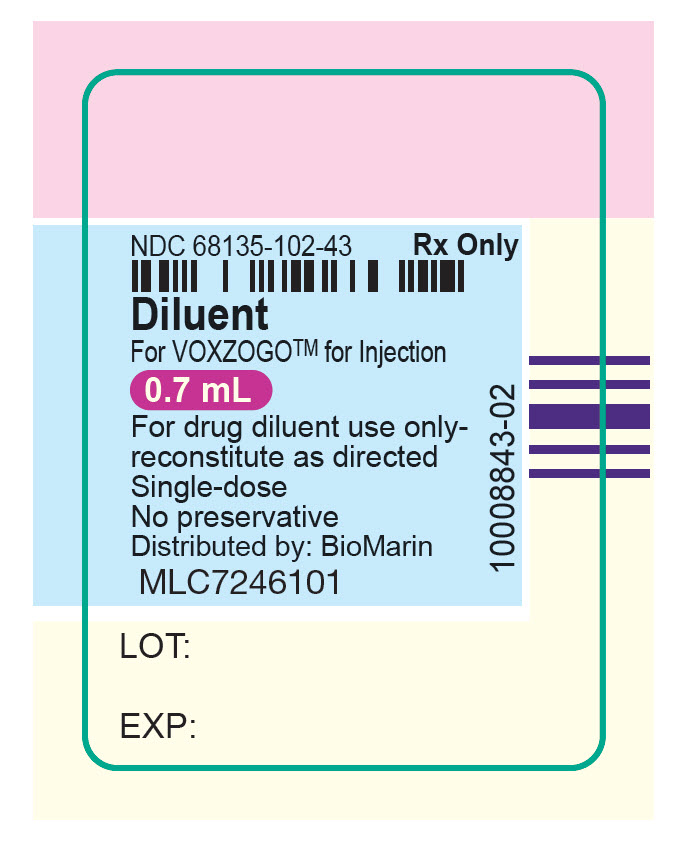
vosoritide injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:68135-094 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VOSORITIDE (UNII: 7SE5582Q2P) (VOSORITIDE - UNII:7SE5582Q2P) VOSORITIDE 0.56 mg in 0.7 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.035 mg in 0.7 mL Product Characteristics Color WHITE (white to yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-094-84 10 in 1 CARTON 1 0.7 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214938 11/19/2021 Part 2 of 2 0.7ML DILUENT FOR 0.56MG VOXOGO KIT
water injection, solutionProduct Information Item Code (Source) NDC:68135-102 Route of Administration SUBCUTANEOUS Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-102-43 10 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214938 11/19/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214938 11/19/2021 VOXZOGO 1.2MG
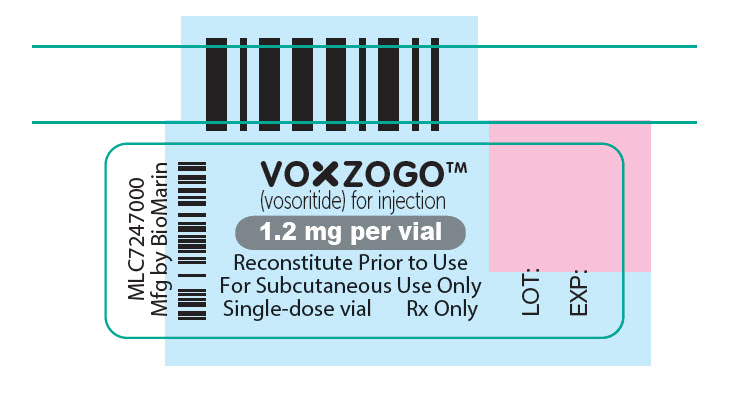
vosoritide kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68135-181 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-181-93 1 in 1 CARTON 11/19/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 VIAL, SINGLE-DOSE 6 mL Part 2 1 CARTON 10 Part 1 of 2 VOXZOGO 1.2MG VIAL
vosoritide injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:68135-130 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VOSORITIDE (UNII: 7SE5582Q2P) (VOSORITIDE - UNII:7SE5582Q2P) VOSORITIDE 1.2 mg in 0.6 mL Inactive Ingredients Ingredient Name Strength SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) 0.65 mg in 0.6 mL Product Characteristics Color WHITE (white to yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-130-92 10 in 1 CARTON 1 0.6 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214938 11/19/2021 Part 2 of 2 0.6ML DILUENT FOR 1.2MG VOXOGO KIT
water injection, solutionProduct Information Item Code (Source) NDC:68135-158 Route of Administration SUBCUTANEOUS Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68135-158-17 10 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214938 11/19/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214938 11/19/2021 Labeler - BioMarin Pharmaceutical Inc. (079722386) Establishment Name Address ID/FEI Business Operations Vetter Pharma-Fertigung Gmbh & Co. KG 312670654 ANALYSIS(68135-082, 68135-119, 68135-181) , MANUFACTURE(68135-082, 68135-119, 68135-181) , PACK(68135-082, 68135-119, 68135-181)