Label: IPRATROPIUM BROMIDE solution
- NDC Code(s): 76204-100-01, 76204-100-25, 76204-100-30, 76204-100-60
- Packager: Ritedose Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
The active ingredient, ipratropium bromide monohydrate, USP, is an anticholinergic bronchodilator chemically described as 8-azoniabicyclo [3.2.1]- octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide, monohydrate (endo, syn)-, (±)-; a synthetic quaternary ammonium compound, chemically related to atropine.
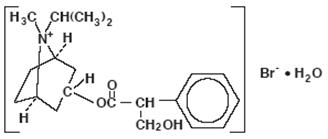
Ipratropium Bromide Monohydrate C 20H 30BrNO 3•H 2O Mol.Wt. 430.4
Ipratropium bromide is a white crystalline substance, freely soluble in water and lower alcohols. It is a quaternary ammonium compound and thus exists in an ionized state in aqueous solutions. It is relatively insoluble in non-polar media.
Ipratropium Bromide Inhalation Solution is administered by oral inhalation with the aid of a nebulizer. It contains ipratropium bromide, USP 0.02% (anhydrous basis) in a sterile, preservative-free, isotonic saline solution, pH-adjusted to 3.4 (3 to 4) with hydrochloric acid.
-
CLINICAL PHARMACOLOGY
Ipratropium bromide is an anticholinergic (parasympatholytic) agent that, based on animal studies, appears to inhibit vagally mediated reflexes by antagonizing the action of acetylcholine, the transmitter agent released from the vagus nerve.
Anticholinergics prevent the increases in intracellular concentration of cyclic guanosine monophosphate (cyclic GMP) that are caused by interaction of acetylcholine with the muscarinic receptor on bronchial smooth muscle.
The bronchodilation following inhalation of ipratropium bromide is primarily a local, site-specific effect, not a systemic one. Much of an administered dose is swallowed but not absorbed, as shown by fecal excretion studies. Following nebulization of a 2 mg dose, a mean 7% of the dose was absorbed into the systemic circulation either from the surface of the lung or from the gastrointestinal tract. The half-life of elimination is about 1.6 hours after intravenous administration. Ipratropium bromide is minimally (0 to 9% in vitro) bound to plasma albumin and a 1-acid glycoproteins. It is partially metabolized. Autoradiographic studies in rats have shown that ipratropium bromide does not penetrate the blood-brain barrier. Ipratropium bromide has not been studied in patients with hepatic or renal insufficiency. It should be used with caution in those patient populations.
In controlled 12-week studies in patients with bronchospasm associated with chronic obstructive pulmonary disease (chronic bronchitis and emphysema) significant improvements in pulmonary function (FEV 1 increases of 15% or more) occurred within 15 to 30 minutes, reached a peak in 1 to 2 hours, and persisted for periods of 4 to 5 hours in the majority of patients, with about 25% to 38% of the patients demonstrating increases of 15% or more for at least 7 to 8 hours. Continued effectiveness of ipratropium bromide inhalation solution was demonstrated throughout the 12-week period. In addition, significant increases in forced vital capacity (FVC) have been demonstrated. However, ipratropium bromide did not consistently produce significant improvement in subjective symptom scores nor in quality of life scores over the 12-week duration of study.
Additional controlled 12-week studies were conducted to evaluate the safety and effectiveness of ipratropium bromide inhalation solution administered concomitantly with the beta adrenergic bronchodilator solutions metaproterenol and albuterol compared with the administration of each of the beta agonists alone. Combined therapy produced significant additional improvement in FEV 1 and FVC. On combined therapy, the median duration of 15% improvement in FEV 1 was 5 to 7 hours, compared with 3 to 4 hours in patients receiving a beta agonist alone.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
The use of ipratropium bromide inhalation solution as a single agent for the relief of bronchospasm in acute COPD exacerbation has not been adequately studied. Drugs with faster onset of action may be preferable as initial therapy in this situation. Combination of ipratropium bromide and beta agonists has not been shown to be more effective than either drug alone in reversing the bronchospasm associated with acute COPD exacerbation.
Immediate hypersensitivity reactions may occur after administration of ipratropium bromide, as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm and oropharyngeal edema.
-
PRECAUTIONS
General
Ipratropium bromide should be used with caution in patients with narrow-angle glaucoma, prostatic hypertrophy or bladder-neck obstruction.
Information for Patients
Patients should be advised that temporary blurring of vision, precipitation or worsening of narrow-angle glaucoma or eye pain may result if the solution comes into direct contact with the eyes. Use of a nebulizer with mouthpiece rather than face mask may be preferable, to reduce the likelihood of the nebulizer solution reaching the eyes. Patients should be advised that ipratropium bromide inhalation solution can be mixed in the nebulizer with albuterol or metaproterenol if used within one hour. Drug stability and safety of Ipratropium Bromide Inhalation Solution when mixed with other drugs in a nebulizer have not been established. Patients should be reminded that ipratropium bromide inhalation solution should be used consistently as prescribed throughout the course of therapy.
Drug Interactions
Ipratropium bromide has been shown to be a safe and effective bronchodilator when used in conjunction with beta adrenergic bronchodilators. Ipratropium bromide has also been used with other pulmonary medications, including methylxanthines and corticosteroids, without adverse drug interactions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year oral carcinogenicity studies in rats and mice have revealed no carcinogenic potential at dietary doses up to 6 mg/kg/day of ipratropium bromide.
Results of various mutagenicity studies (Ames test, mouse dominant lethal test, mouse micronucleus test and chromosome aberration of bone marrow in Chinese hamsters) were negative.
Fertility of male or female rats at oral doses up to 50 mg/kg/day was unaffected by ipratropium bromide administration. At doses above 90 mg/kg increased resorption and decreased conception rates were observed.
Pregnancy
TERATOGENIC EFFECTS
Pregnancy Category B
Oral reproduction studies performed in mice, rats and rabbits at doses of 10, 100, and 125 mg/kg respectively, and inhalation reproduction studies in rats and rabbits at doses of 1.5 and 1.8 mg/kg (or approximately 38 and 45 times the recommended human daily dose) respectively, have demonstrated no evidence of teratogenic effects as a result of ipratropium bromide. However, no adequate or well-controlled studies have been conducted in pregnant women. Because animal reproduction studies are not always predictive of human response, ipratropium bromide should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether ipratropium bromide is excreted in human milk. Although lipid-insoluble quaternary bases pass into breast milk, it is unlikely that ipratropium bromide would reach the infant to a significant extent, especially when taken by inhalation since ipratropium bromide is not well absorbed systemically after inhalation or oral administration. However, because many drugs are excreted in human milk, caution should be exercised when ipratropium bromide is administered to a nursing woman.
-
ADVERSE REACTIONS
Adverse reaction information concerning ipratropium bromide inhalation solution is derived from 12-week active-controlled clinical trials. Additional information is derived from foreign post-marketing experience and the published literature.
All adverse events, regardless of drug relationship, reported by three percent or more patients in the 12-week controlled clinical trials appear in the table below.
Additional adverse reactions reported in less than three percent of the patients treated with ipratropium bromide include tachycardia, palpitations, eye pain, urinary retention, urinary tract infection and urticaria. Cases of precipitation or worsening of narrow-angle glaucoma, mydriasis, and acute eye pain have been reported.
Lower respiratory adverse reactions (bronchitis, dyspnea and bronchospasm) were the most common events leading to discontinuation of ipratropium bromide therapy in the 12-week trials. Headache, mouth dryness and aggravation of COPD symptoms are more common when the total daily dose of ipratropium bromide equals or exceeds 2,000 mcg.
Allergic-type reactions such as skin-rash, angioedema of tongue, lips and face, urticaria, laryngospasm and anaphylactic reaction have been reported. Many of the patients had a history of allergies to other drugs and/or foods.
All Adverse Events, from a Double-blind, Parallel, 12-week Study of patients with COPD * PERCENT OF PATIENTS Ipratropium Metaproterenol Ipratropium/
MetaproterenolAlbuterol Ipratropium/
Albuterol(500 mcg t.i.d.) (15 mg t.i.d.) (500 mcg t.i.d./
15 mg t.i.d.)(2.5 mg t.i.d.) (500 mcg t.i.d./
2.5 mg t.i.d.)n = 219 n = 212 n = 108 n = 205 n = 100 - *
- All adverse events, regardless of drug relationship, reported by three percent or more patients in the 12-week controlled clinical trials.
Body as a Whole-General Disorders Headache 6.4 5.2 6.5 6.3 9.0 Pain 4.1 3.3 0.9 2.9 5.0 Influenza-like symptoms 3.7 4.7 6.5 0.5 1.0 Back Pain 3.2 1.9 1.9 2.4 0.0 Chest Pain 3.2 4.2 5.6 2.0 1.0 Cardiovascular Disorders Hypertension/Hypertension Aggravated 0.9 1.9 0.9 1.5 4.0 Central & Peripheral Nervous System Dizziness 2.3 3.3 1.9 3.9 4.0 Insomnia 0.9 0.5 4.6 1.0 1.0 Tremor 0.9 7.1 8.3 1.0 0.0 Nervousness 0.5 4.7 6.5 1.0 1.0 Gastrointestinal System Disorders Mouth Dryness 3.2 0.0 1.9 2.0 3.0 Nausea 4.1 3.8 1.9 2.9 2.0 Constipation 0.9 0.0 3.7 1.0 1.0 Musculo-skeletal System Disorders Arthritis 0.9 1.4 0.9 0.5 3.0 Respiratory System Disorders (Lower) Coughing 4.6 8.0 6.5 5.4 6.0 Dyspnea 9.6 13.2 16.7 12.7 9.0 Bronchitis 14.6 24.5 15.7 16.6 20.0 Bronchospasm 2.3 2.8 4.6 5.4 5.0 Sputum Increased 1.4 1.4 4.6 3.4 0.0 Respiratory Disorder 0.0 6.1 6.5 2.0 4.0 Respiratory System Disorders (Upper) Upper Respiratory Tract Infection 13.2 11.3 9.3 12.2 16.0 Pharyngitis 3.7 4.2 5.6 2.9 4.0 Rhinitis 2.3 4.2 1.9 2.4 0.0 Sinusitus 2.3 2.8 0.9 5.4 4.0 -
OVERDOSAGE
Acute systemic overdosage by inhalation solution is unlikely since ipratropium bromide is not well absorbed after inhalation at up to four-fold the recommended dose, or after oral administration at up to forty-fold the recommended dose. The oral LD 50 of ipratropium bromide ranged between 1001 and 2010 mg/kg in mice; between 1667 and 4000 mg/kg in rats; and between 400 and 1300 mg/kg in dogs.
-
DOSAGE AND ADMINISTRATION
The usual dosage of ipratropium bromide inhalation solution is 500 mcg (1 Unit-Dose Vial) administered three to four times a day by oral nebulization, with doses 6 to 8 hours apart. Ipratropium bromide inhalation solution unit-dose vials contain 500 mcg ipratropium bromide, USP anhydrous in 2.5 mL normal saline. Ipratropium bromide inhalation solution can be mixed in the nebulizer with albuterol or metaproterenol if used within one hour. Drug stability and safety of Ipratropium Bromide Inhalation Solution when mixed with other drugs in a nebulizer have not been established.
-
HOW SUPPLIED
Ipratropium Bromide Inhalation Solution Unit Dose Vial is supplied as a 0.02% clear, colorless solution containing 2.5 mL.
NDC 76204-100-25, 25 vials per carton / 25 vials per foil pouch
NDC 76204-100-30, 30 vials per carton / 30 vials per foil pouch
NDC 76204-100-01, 30 vials per carton / 1 vial per foil pouch
NDC 76204-100-60, 60 vials per carton / 30 vials per foil pouch
Each vial is made from a low density polyethylene (LDPE) resin.
Vials are supplied in a foil pouch.
-
SPL UNCLASSIFIED SECTION
ATTENTION PHARMACIST: Detach "Patient's Instructions for Use" from Package Insert and dispense with solution.
Rx Only
Manufactured by:
The Ritedose Corporation
Columbia, SC 29203 for
Ritedose Pharmaceuticals, LLC
Columbia, SC 29203To report SUSPECTED ADVERSE
REACTIONS, contact Ritedose
Pharmaceuticals, LLC
at 1-855-806-3300 or FDA
at 1-800-FDA-1088 or www.fda.gov/medwatchRPIN0044
JAN 2013 -
Patient's Instructions for Use
Ipratropium Bromide
Inhalation Solution 0.02%
Read complete instructions carefully before using.
- Twist open the top of one unit dose vial and squeeze the contents into the nebulizer reservoir. (Figure 1).
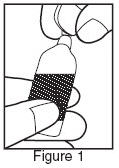
- Connect the nebulizer reservoir to the mouthpiece or face mask (Figure 2).
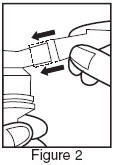
- Connect the nebulizer to the compressor.
- Sit in a comfortable, upright position; place the mouthpiece in your mouth (Figure 3) or put on the face mask and turn on the compressor. If a face mask is used, care should be taken to avoid leakage around the mask as temporary blurring of vision, pupil enlargement, precipitation or worsening of narrow angle glaucoma, or eye pain may occur if the solution comes into direct contact with the eyes.
- Breathe as calmly, deeply, and evenly as possible until no more mist is formed in the nebulizer chamber (about 5 to 15 minutes). At this point, the treatment is finished.
- Clean the nebulizer (see manufacturer's instructions).
Note: Use only as directed by your physician. More frequent administration or higher doses are not recommended. Ipratropium Bromide Inhalation Solution can be mixed in the nebulizer with albuterol or metaproterenol if used within one hour but not with other drugs. Drug stability and safety of Ipratropium Bromide Inhalation Solution when mixed with other drugs in the nebulizer have not been established.
Store between 59°F (15°C) and 86°F (30°). Protect from light. Store unused vials in the foil pouch.
ADDITIONAL INSTRUCTIONS:
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________
____________________________________ - Twist open the top of one unit dose vial and squeeze the contents into the nebulizer reservoir. (Figure 1).
- SPL UNCLASSIFIED SECTION
-
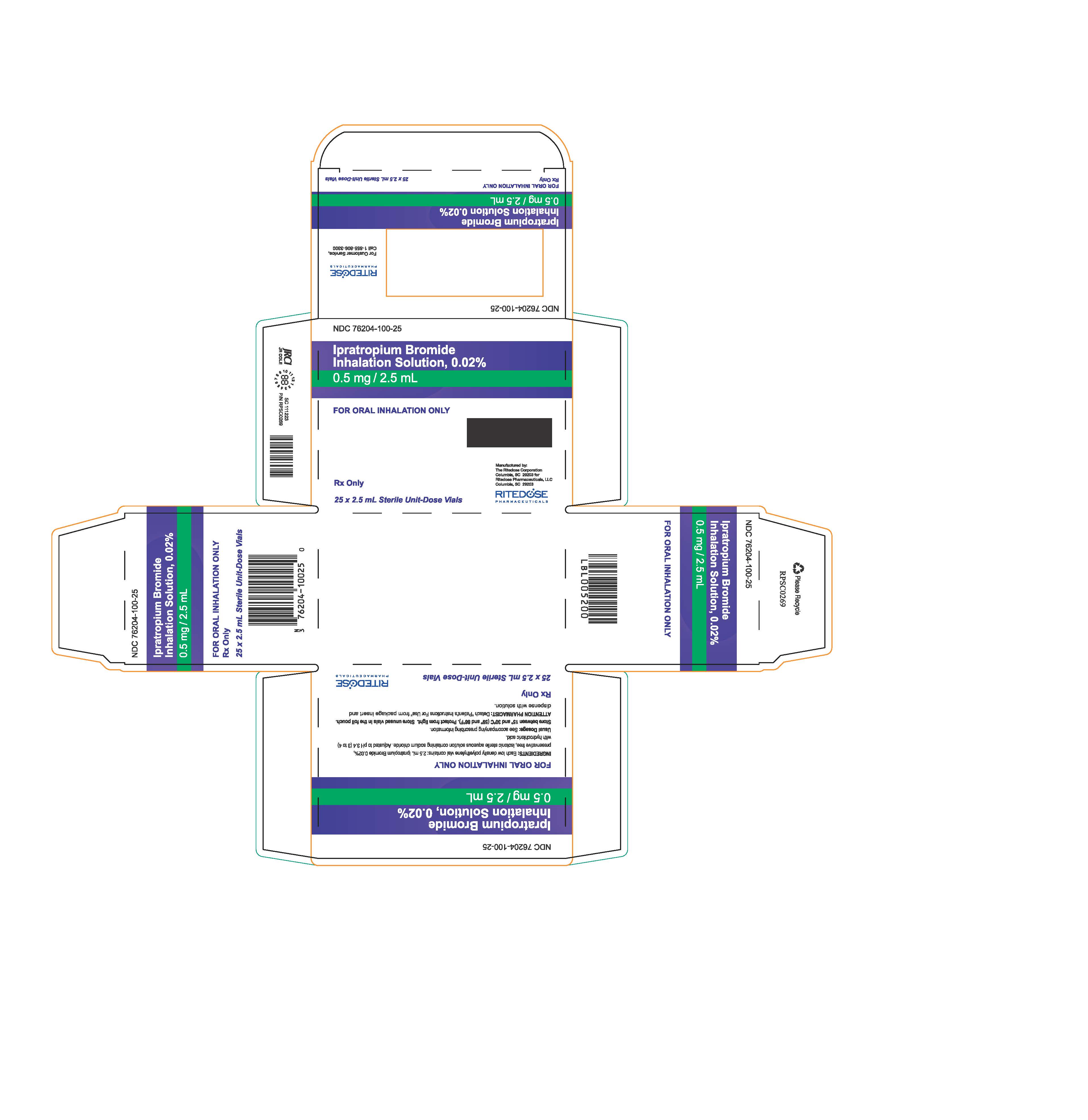
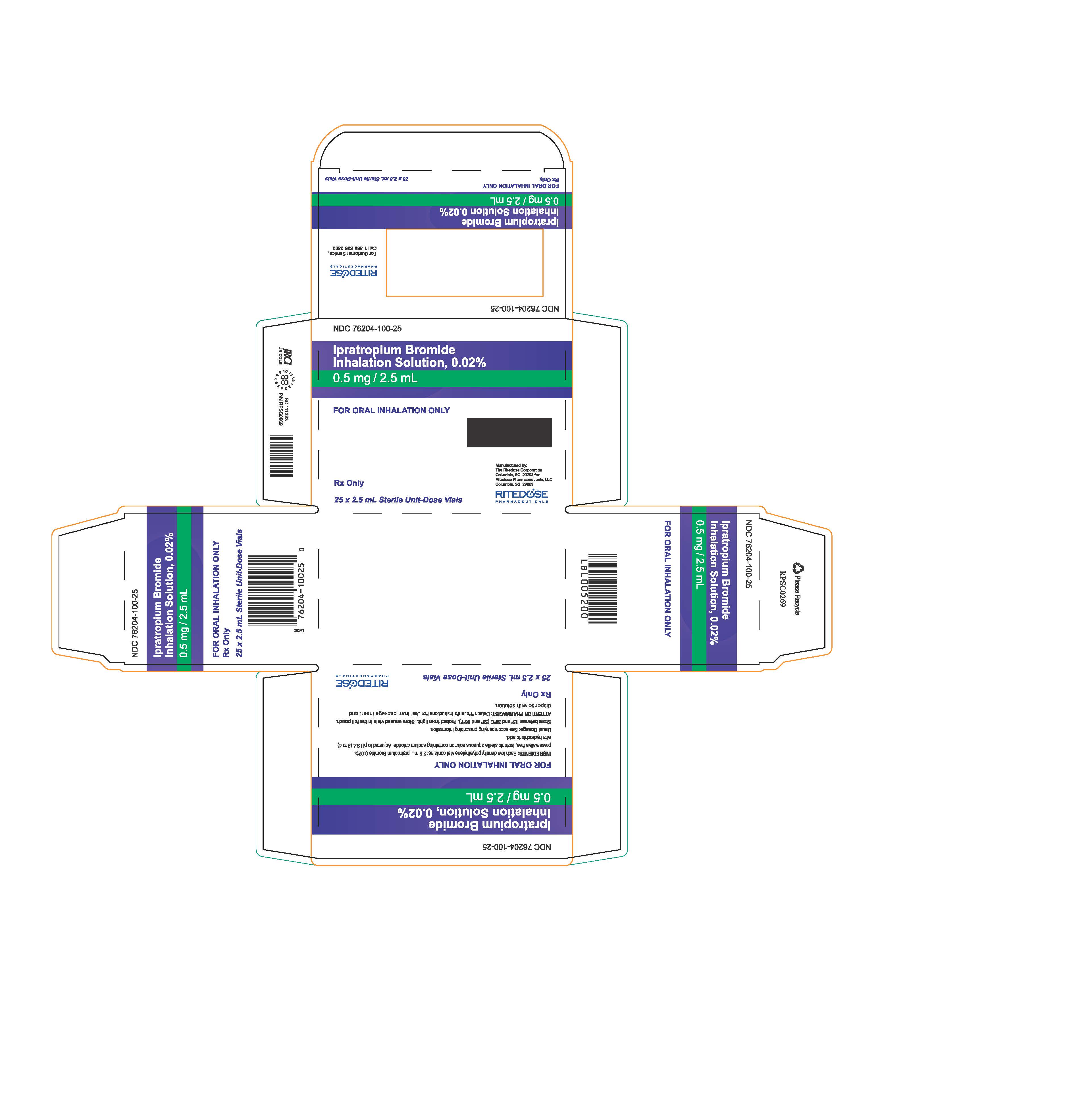
PRINCIPAL DISPLAY PANEL - RDP 25 ct Brick Carton
NDC 76204-100-25
Ipratropium Bromide Inhalation Solution, 0.02%
0.5 mg / 2.5 mLFOR ORAL INHALATION ONLY
INGREDIENTS: Each low density polyethylene vial contains: 2.5 mL Ipratropium Bromide 0.02%, preservative free, isotonic sterile aqueous solution containing sodium chloride. Adjusted to pH 3.4 (3 to 4) with hydrochloric acid.
Usual Dosage: See accompanying prescribing information.
Store between 15° and 30°C (59° and 86°F). Protect from light. Store unused vials in the foil pouch.
ATTENTION PHARMACIST: Detach "Patient's Instructions For Use" from package insert and dispense with solution.Rx Only
25 x 2.5 mL Sterile Unit-Dose Vials
-
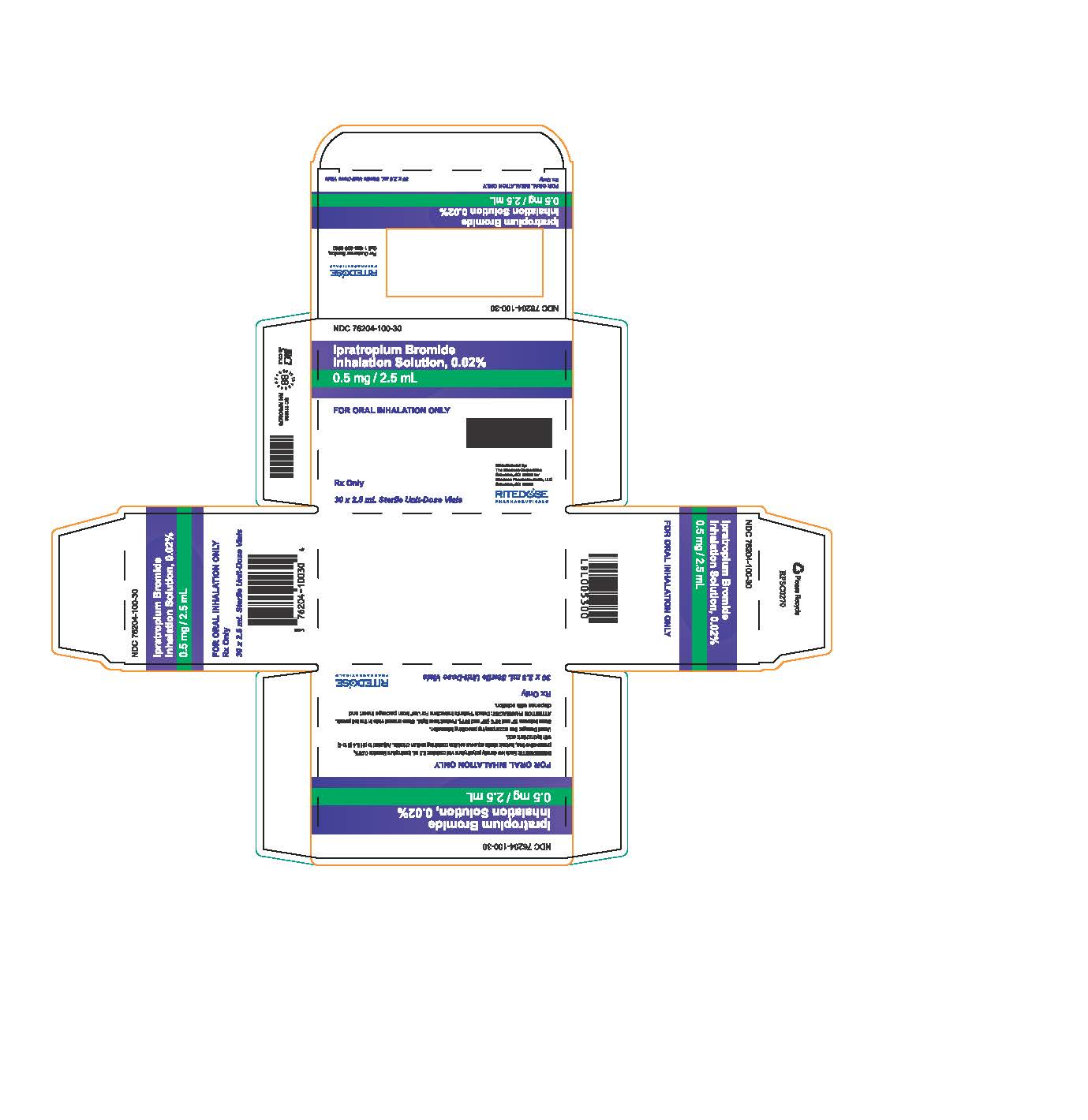
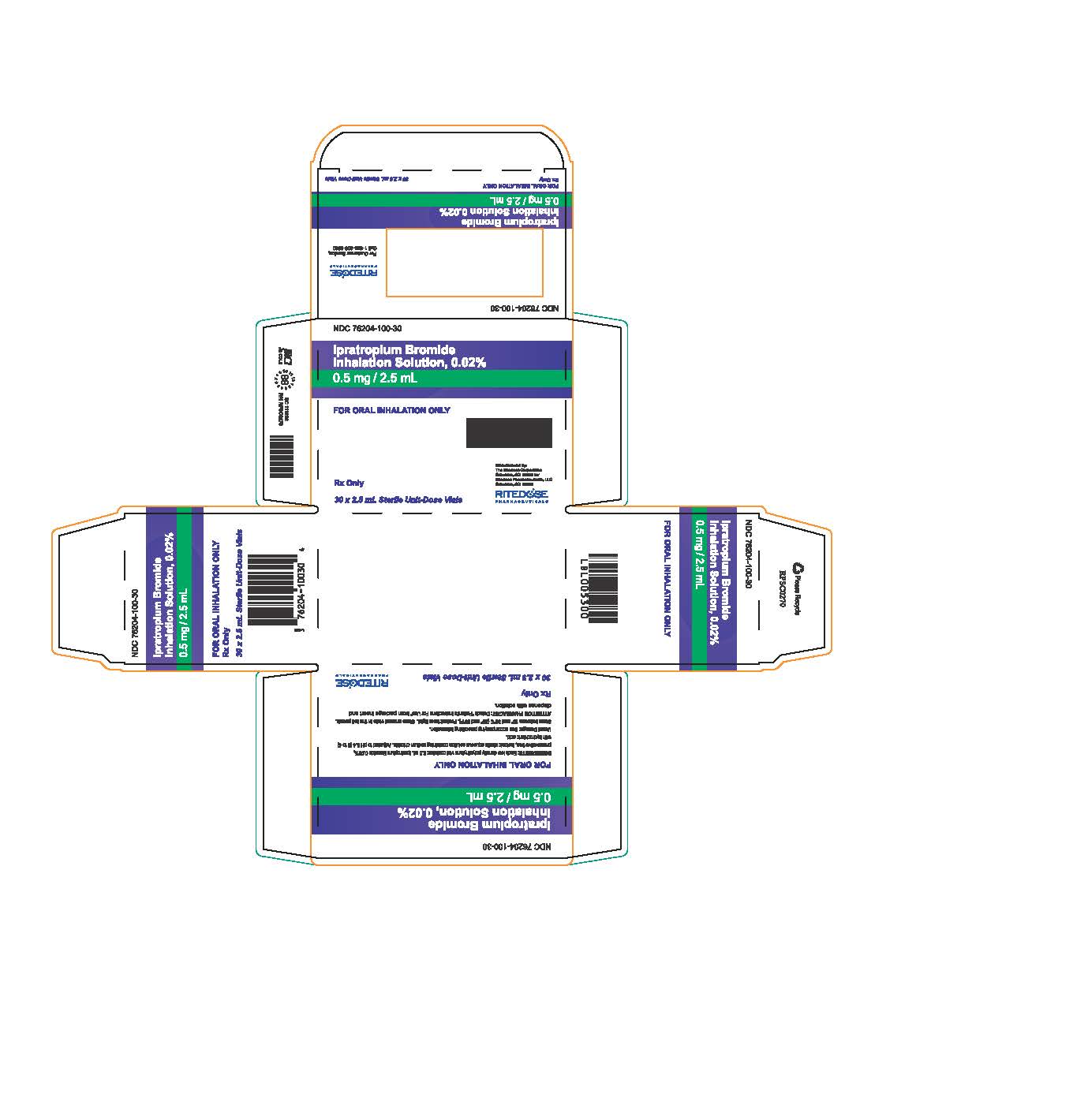
PRINCIPAL DISPLAY PANEL - RDP 30 ct Brick Carton
NDC 76204-100-30
Ipratropium Bromide Inhalation Solution, 0.02%
0.5 mg / 2.5 mLFOR ORAL INHALATION ONLY
INGREDIENTS: Each low density polyethylene vial contains: 2.5 mL Ipratropium Bromide 0.02%, preservative free, isotonic sterile aqueous solution containing sodium chloride. Adjusted to pH 3.4 (3 to 4) with hydrochloric acid.
Usual Dosage: See accompanying prescribing information.
Store between 15° and 30°C (59° and 86°F). Protect from light. Store unused vials in the foil pouch.
ATTENTION PHARMACIST: Detach "Patient's Instructions For Use" from package insert and dispense with solution.Rx Only
30 x 2.5 mL Sterile Unit-Dose Vials
-
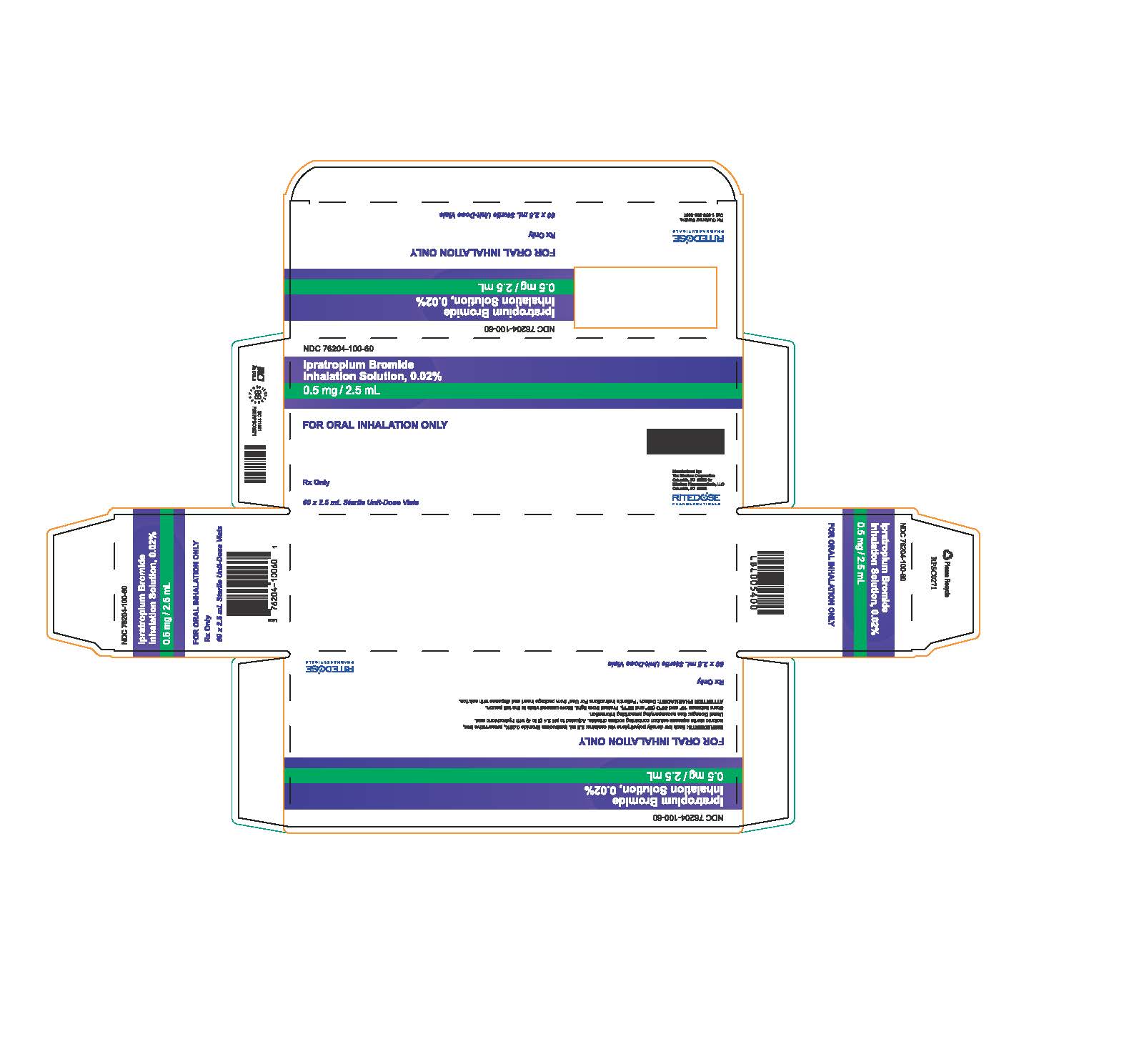
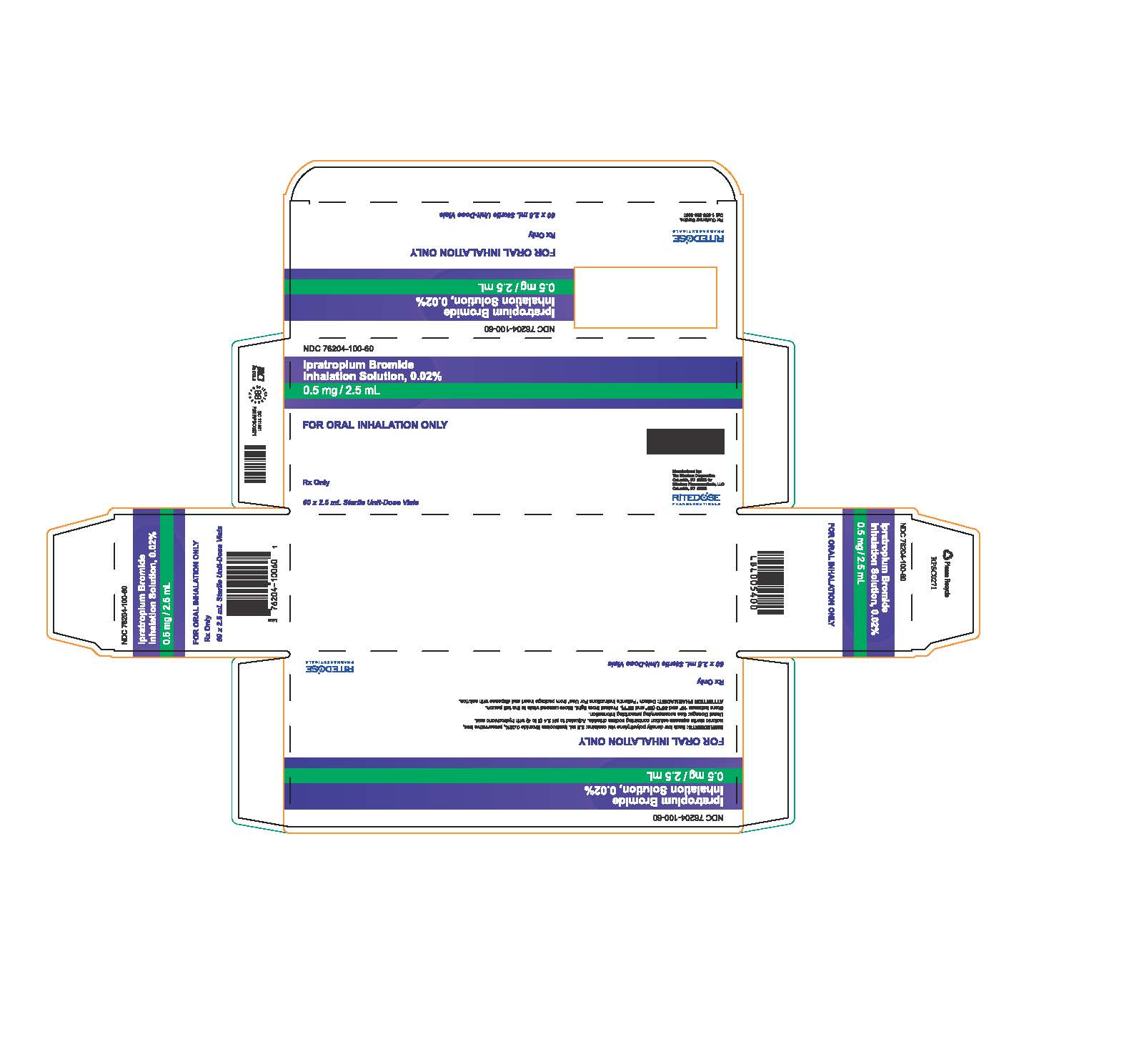
PRINCIPAL DISPLAY PANEL - RDP 60 ct Brick Carton
NDC 76204-100-60
Ipratropium Bromide Inhalation Solution, 0.02%
0.5 mg / 2.5 mLFOR ORAL INHALATION ONLY
INGREDIENTS: Each low density polyethylene vial contains: 2.5 mL Ipratropium Bromide 0.02%, preservative free, isotonic sterile aqueous solution containing sodium chloride. Adjusted to pH 3.4 (3 to 4) with hydrochloric acid.
Usual Dosage: See accompanying prescribing information.
Store between 15° and 30°C (59° and 86°F). Protect from light. Store unused vials in the foil pouch.
ATTENTION PHARMACIST: Detach "Patient's Instructions For Use" from package insert and dispense with solution.Rx Only
60 x 2.5 mL Sterile Unit-Dose Vials
-
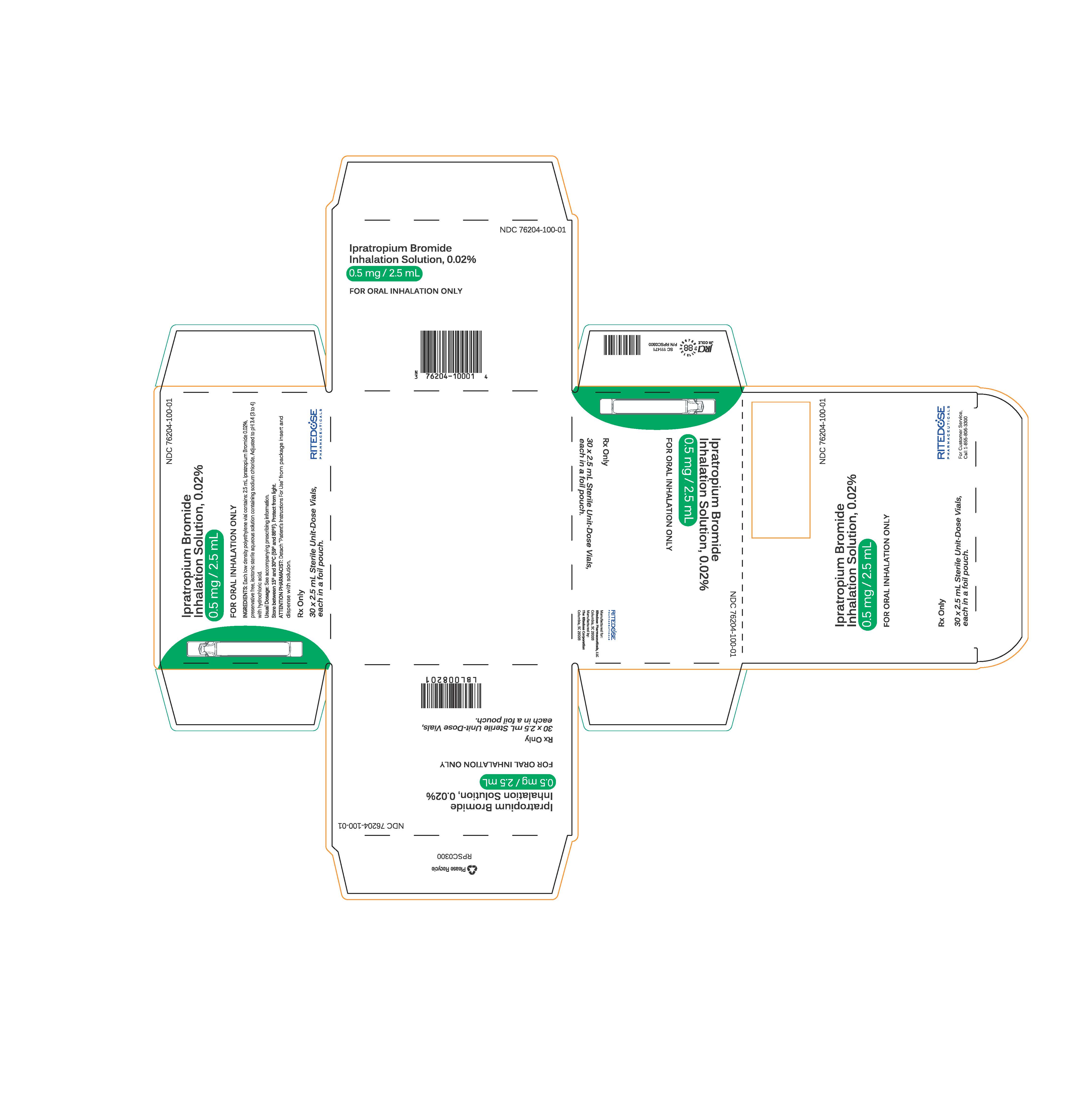
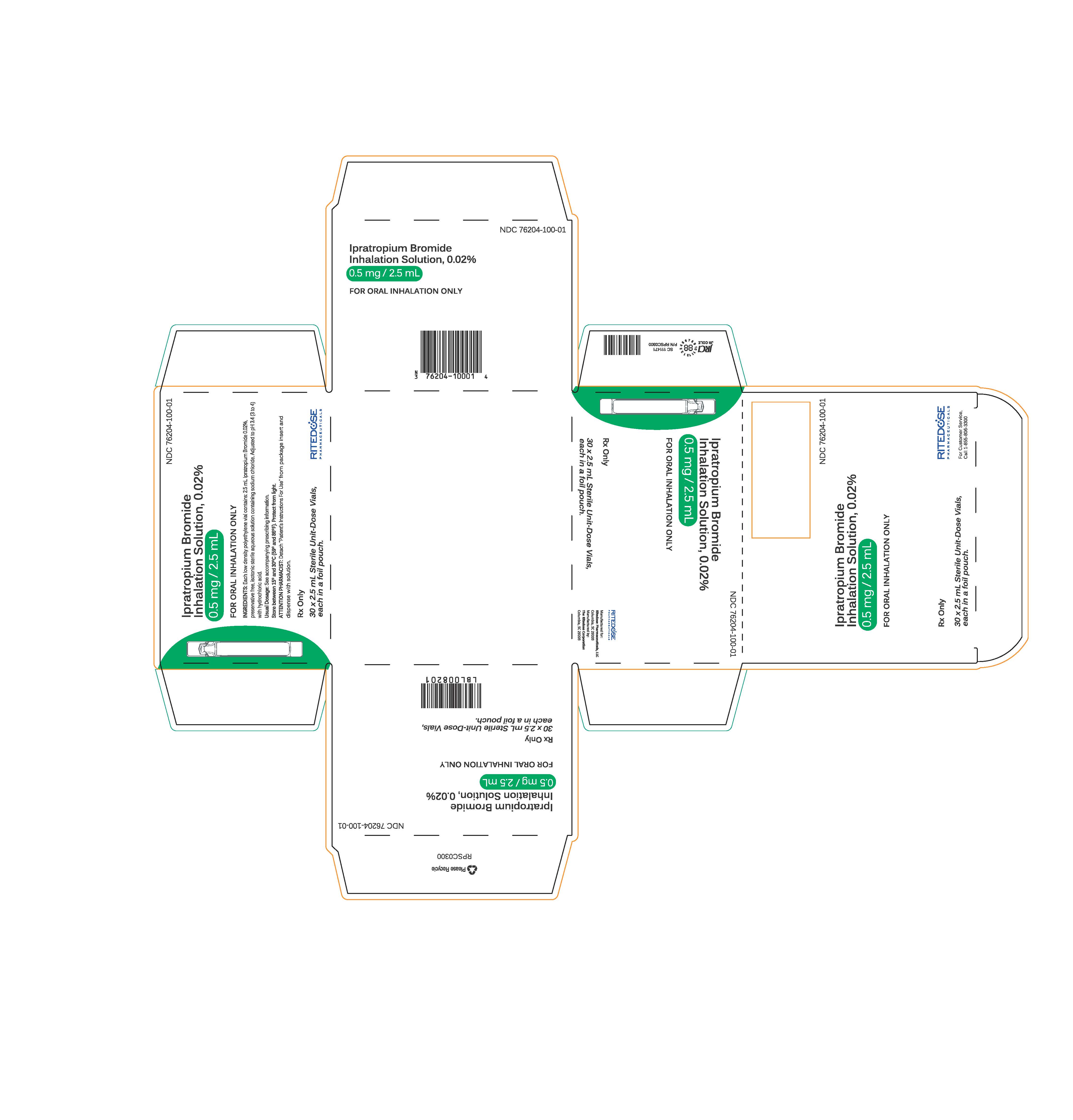
PRINCIPAL DISPLAY PANEL - RDP 30 ct Singles Carton
NDC 76204-100-01
Ipratropium Bromide Inhalation Solution, 0.02%
0.5 mg / 2.5 mLFOR ORAL INHALATION ONLY
INGREDIENTS: Each low density polyethylene vial contains: 2.5 mL Ipratropium Bromide 0.02%, preservative free, isotonic sterile aqueous solution containing sodium chloride. Adjusted to pH 3.4 (3 to 4) with hydrochloric acid.
Usual Dosage: See accompanying prescribing information.
Store between 15° and 30°C (59° and 86°F). Protect from light.
ATTENTION PHARMACIST: Detach "Patient's Instructions For Use" from package insert and dispense with solution.Rx Only
30 x 2.5 mL Sterile Unit-Dose Vials, each in a foil pouch
-
INGREDIENTS AND APPEARANCE
IPRATROPIUM BROMIDE
ipratropium bromide solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76204-100 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IPRATROPIUM BROMIDE (UNII: J697UZ2A9J) (IPRATROPIUM - UNII:GR88G0I6UL) IPRATROPIUM BROMIDE ANHYDROUS 0.5 mg in 2.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76204-100-25 1 in 1 CARTON 11/21/2011 1 25 in 1 POUCH 1 2.5 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC:76204-100-30 1 in 1 CARTON 11/21/2011 2 30 in 1 POUCH 2 2.5 mL in 1 AMPULE; Type 0: Not a Combination Product 3 NDC:76204-100-60 2 in 1 CARTON 11/21/2011 3 30 in 1 POUCH 3 2.5 mL in 1 AMPULE; Type 0: Not a Combination Product 4 NDC:76204-100-01 30 in 1 CARTON 11/21/2011 4 1 in 1 POUCH 4 2.5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075693 02/01/2011 Labeler - Ritedose Pharmaceuticals, LLC (968062294)