Label: BREVIBLOC- esmolol hydrochloride injection
- NDC Code(s): 10019-668-10, 10019-672-10
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BREVIBLOC injection safely and effectively. See full prescribing information for BREVIBLOC injection. BREVIBLOC (Esmolol ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE 1.1 Supraventricular Tachycardia or Noncompensatory Sinus Tachycardia - BREVIBLOC (Esmolol Hydrochloride) injection is indicated for the rapid control of ventricular rate in patients with atrial ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Dosing for the Treatment of Supraventricular Tachycardia or Noncompensatory Sinus Tachycardia - BREVIBLOC injection is administered by continuous intravenous infusion with or without a ...
-
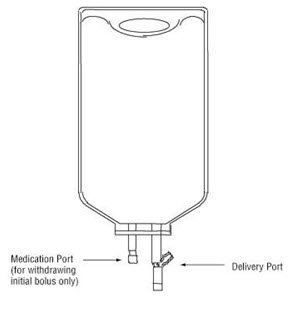
3 DOSAGE FORMS AND STRENGTHS All BREVIBLOC injection dosage forms are iso-osmotic solutions of esmolol hydrochloride in sodium chloride. Table 2 BREVIBLOC Injection Presentations - Product Name - BREVIBLOC - PREMIXED ...
-
4 CONTRAINDICATIONS BREVIBLOC (Esmolol Hydrochloride) injection is contraindicated in patients with: • Severe sinus bradycardia: May precipitate or worsen bradycardia resulting in cardiogenic shock and cardiac ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypotension - Hypotension can occur at any dose but is dose-related. Patients with hemodynamic compromise or on interacting medications are at particular risk. Severe reactions may include ...
-
6 ADVERSE REACTIONS 6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS Concomitant use of BREVIBLOC injection with other drugs that can lower blood pressure, reduce myocardial contractility, or interfere with sinus node function or electrical impulse propagation in ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Esmolol hydrochloride has been shown to produce increased fetal resorptions with minimal maternal toxicity in rabbits when given in doses approximately 8 times the maximum human ...
-
10 OVERDOSAGE 10.1 Signs and Symptoms of Overdose - Overdoses of BREVIBLOC (Esmolol Hydrochloride) injection can cause cardiac and central nervous system effects. These effects may precipitate severe signs ...
-
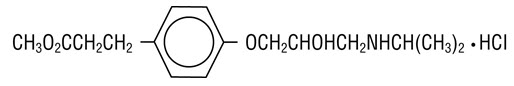
11 DESCRIPTION BREVIBLOC (Esmolol Hydrochloride) injection is a beta adrenergic receptor blocker with a very short duration of action (elimination half-life is approximately 9 minutes). Esmolol hydrochloride ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - BREVIBLOC (Esmolol Hydrochloride) injection is a beta1-selective (cardioselective) adrenergic receptor blocking agent with rapid onset, a very short duration of ...
-
13 NONCLINICAL TOXICOLOGY Because of its short-term usage, no carcinogenicity, mutagenicity, or reproductive performance studies have been conducted with esmolol.
-
14 CLINICAL STUDIES Supraventricular Tachycardia - In two multicenter, randomized, double-blind, controlled comparisons of BREVIBLOC injection with placebo and propranolol, maintenance doses of 50 to 300 mcg/kg/min ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - BREVIBLOC PREMIXED Injection - • NDC 10019-672-10, 2500 mg / 250 mL (10 mg/mL) Ready-to-use INTRAVIA Bags - BREVIBLOC PREMIXED Double Strength Injection - • NDC 10019-668-10 ...
-
17 PATIENT COUNSELING INFORMATION Physicians should inform patients of the risks associated with BREVIBLOC injection: • The most common adverse reactions are symptomatic hypotension (hyperhidrosis, dizziness) and asymptomatic ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Container Label - LOT EXP - NDC 10019-668-10 - BREVIBLOC - PREMIXED - DOUBLE STRENGTH - Injection - Esmolol Hydrochloride in Sodium Chloride - 2,000 mg/100 mL (20 mg/mL) 100 mL - Iso-Osmotic • No ...
-
INGREDIENTS AND APPEARANCEProduct Information