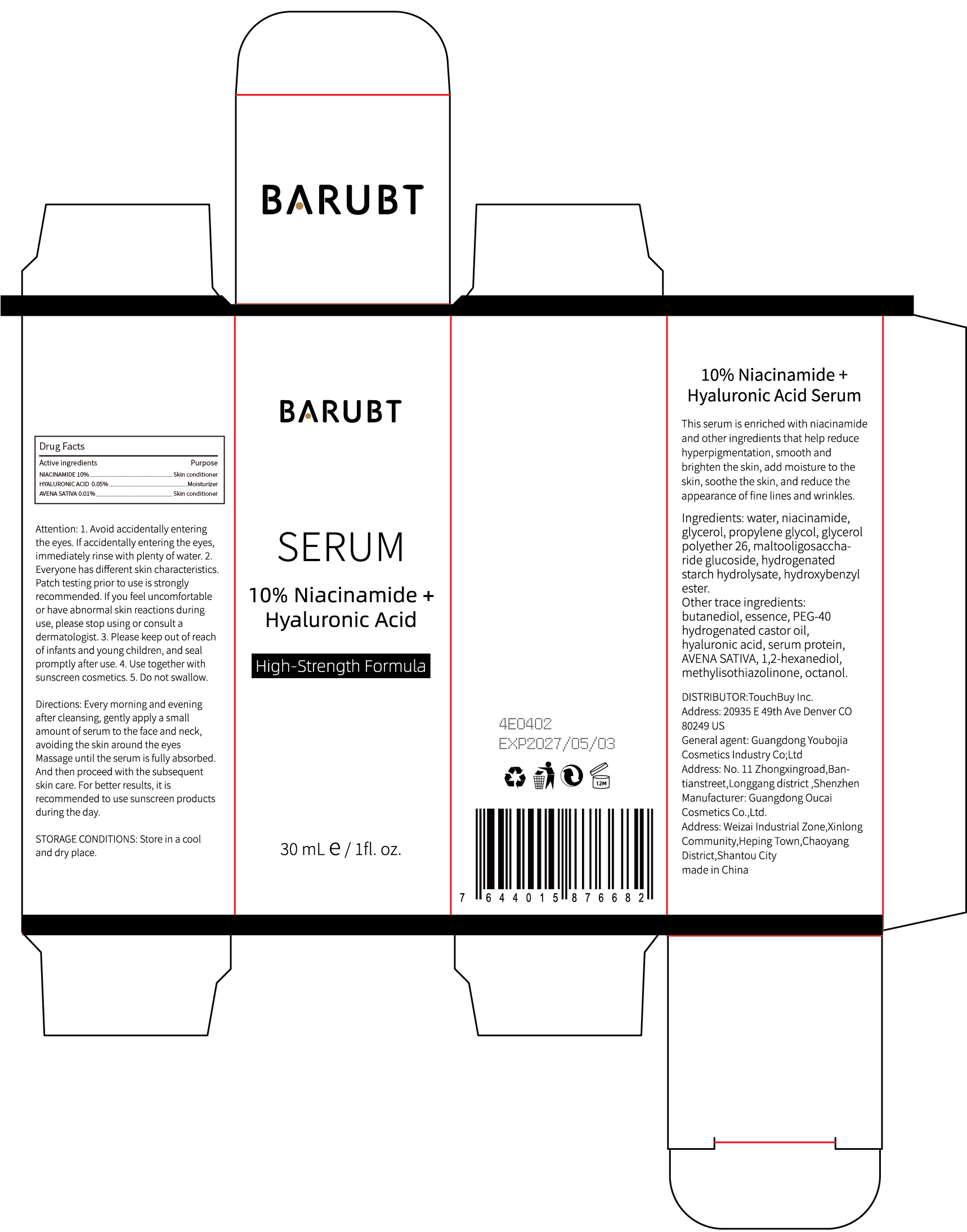
Label: BARUBT NIACINAMIDE HYALURONIC ACID SERUM- niacinamide hyaluronic acid serum lotion
- NDC Code(s): 84712-009-01
- Packager: Guangdong Youbaijia Cosmetic Industry Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- DO NOT USE
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- WHEN USING
-
DOSAGE & ADMINISTRATION
Every morning and evening after cleansing, gently apply a small amount of serum to the face and neck, avoiding the skin around the eyes
Massage until the serum is fully absorbed. And then proceed with the subsequent skin care. For better results, it is recommended to use sunscreen products during the day. - QUESTIONS
- WARNINGS
- INDICATIONS & USAGE
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BARUBT NIACINAMIDE HYALURONIC ACID SERUM
niacinamide hyaluronic acid serum lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84712-009 Route of Administration CUTANEOUS, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVENA SATIVA WHOLE (UNII: 5P8D0Z74RG) (AVENA SATIVA WHOLE - UNII:5P8D0Z74RG) AVENA SATIVA WHOLE 0.003 g in 30 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 3 g in 30 mL HYALURONIC ACID (UNII: S270N0TRQY) (HYALURONIC ACID - UNII:S270N0TRQY) HYALURONIC ACID 0.015 g in 30 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) 0.015 g in 30 mL HYDROGENATED STARCH HYDROLYSATE (UNII: 27F77DSJ5V) 0.003 g in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84712-009-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/12/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 09/12/2024 Labeler - Guangdong Youbaijia Cosmetic Industry Co., Ltd (702314361) Establishment Name Address ID/FEI Business Operations Guangdong Youbaijia Cosmetic Industry Co., Ltd 702314361 manufacture(84712-009) , label(84712-009)