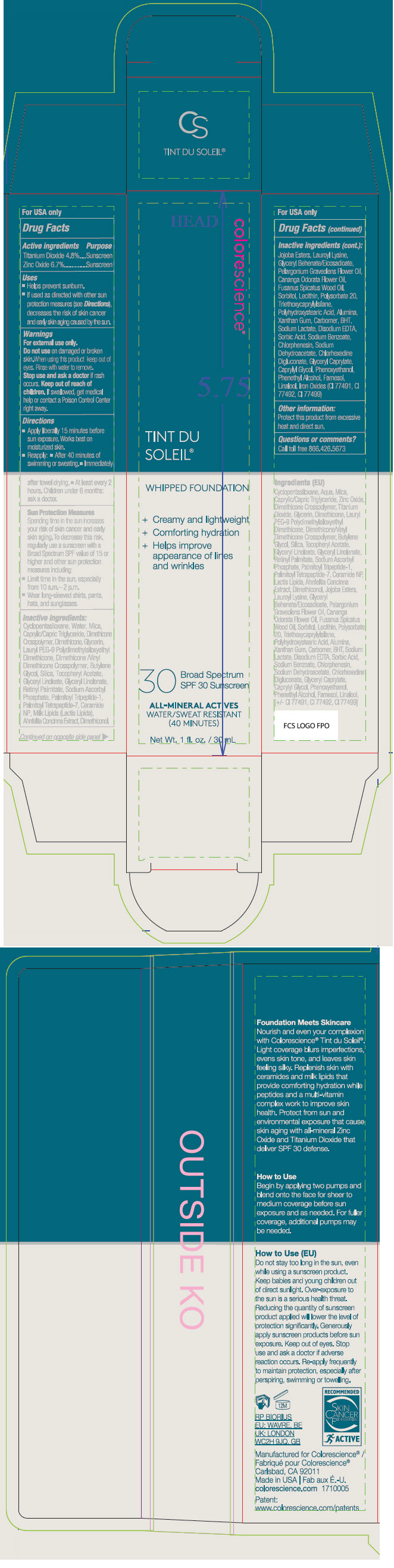
Label: SUNFORGETTABLE TINT DU SOLEIL FOUNDATION SPF 30 SUNSCREEN- titanium dioxide and zinc oxide liquid
- NDC Code(s): 68078-004-02, 68078-004-05
- Packager: Colorescience
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure. Works best on moisturized skin.
- Reapply:
- After 40 minutes of swimming or sweating.
- Immediately after towel drying.
- At least every 2 hours. Children under 6 months: ask a doctor.
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.– 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
-
Inactive ingredients
Cyclopentasiloxane, Water, Mica, Caprylic/Capric Triglyceride, Dimethicone Crosspolymer, Dimethicone, Glycerin, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Dimethicone/Vinyl Dimethicone Crosspolymer, Butylene Glycol, Caprylyl Glycol, Dimethiconol, Silica, Jojoba Esters, Lauroyl Lysine, Polyhydroxystearic Acid, Ahnfeltia Concinna Extract, Glyceryl Behenate/Eicosadioate, Alumina, Sodium Lauroyl Lactylate, Phenethyl Alcohol, Tocopheryl Acetate, Cananga Odorata Flower Oil, Carbomer, Sodium Lactate, Sodium Ascorbyl Phosphate, Fusanus Spicatus Wood Oil, Polysorbate 20, Ceramide NP, Ceramide AP, Ceramide EOP, Triethoxycaprylylsilane, Phytosphingosine, Cholesterol, Xanthan Gum, Ethylhexylglycerin, Pelargonium Graveolens Flower Oil, Palmitoyl Tripeptide-1, Palmitoyl Tetrapeptide-7, Phenoxyethanol, Chlorphenesin, Sodium Dehydroacetate, Sodium Benzoate, Sorbic Acid, Linalool, Farnesol, Iron Oxides (CI 77491, CI 77492, CI 77499).
- Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
SUNFORGETTABLE TINT DU SOLEIL FOUNDATION SPF 30 SUNSCREEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68078-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 48 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 67 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MICA (UNII: V8A1AW0880) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LAUROYL LYSINE (UNII: 113171Q70B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) AHNFELTIOPSIS CONCINNA (UNII: SMF2K46G8D) SANTALUM SPICATUM OIL (UNII: H9LVS6REV4) GLYCERYL BEHENATE/EICOSADIOATE (UNII: 73CJJ317SR) ALUMINUM OXIDE (UNII: LMI26O6933) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) YLANG-YLANG OIL (UNII: 8YOY78GNNX) SODIUM LACTATE (UNII: TU7HW0W0QT) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) XANTHAN GUM (UNII: TTV12P4NEE) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SODIUM BENZOATE (UNII: OJ245FE5EU) Sodium Lauroyl Lactylate (UNII: 7243K85WFO) Ceramide AP (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) Phytosphingosine (UNII: GIN46U9Q2Q) Pelargonium Graveolens Flower Oil (UNII: 3K0J1S7QGC) Cholesterol (UNII: 97C5T2UQ7J) Ethylhexylglycerin (UNII: 147D247K3P) LINALOOL, (+/-)- (UNII: D81QY6I88E) Farnesol (UNII: EB41QIU6JL) SORBIC ACID (UNII: X045WJ989B) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) DIMETHICONOL (100000 CST) (UNII: OSA9UP217S) JOJOBA OIL (UNII: 724GKU717M) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68078-004-05 1 in 1 CARTON 10/05/2015 1 30 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:68078-004-02 1 mL in 1 PACKET; Type 0: Not a Combination Product 10/05/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/05/2015 Labeler - Colorescience (128731929)