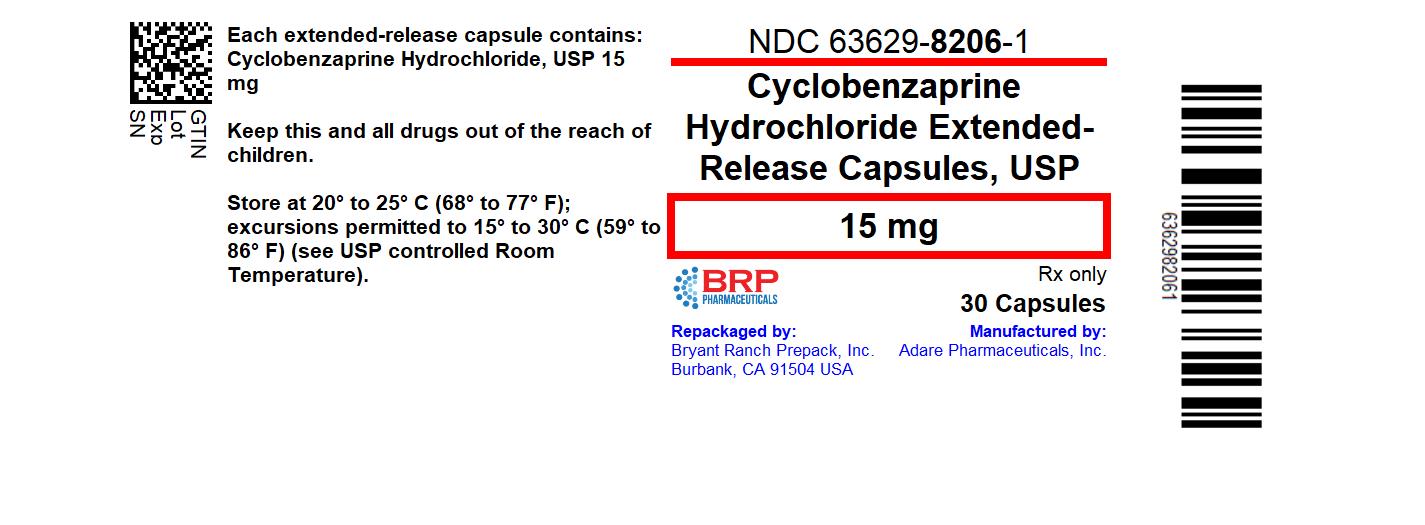
Label: CYCLOBENZAPRINE HYDROCHLORIDE capsule, extended release
- NDC Code(s): 63629-8206-1, 63629-8206-2, 63629-8206-3
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0093-1920
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated March 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use cyclobenzaprine hydrochloride extended-release capsules safely and effectively. See full prescribing information for cyclobenzaprine ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECyclobenzaprine hydrochloride extended-release capsules are indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended adult dose for most patients is one (1) cyclobenzaprine hydrochloride extended-release 15 mg capsule taken once daily. Some patients may require up to 30 mg/day, given as one (1 ...
-
3 DOSAGE FORMS AND STRENGTHSExtended-release capsules in the following strengths: 15 mg: Capsules are orange/orange and are embossed in blue ink with “15 mg” on the body, and Cephalon “C” logo, “Cephalon,” and a dashed band ...
-
4 CONTRAINDICATIONSHypersensitivity to any component of this product. Adverse reactions may include anaphylactic reaction, urticaria, facial and/or tongue swelling, or pruritus. Discontinue cyclobenzaprine ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serotonin Syndrome - The development of a potentially life-threatening serotonin syndrome has been reported with cyclobenzaprine when used in combination with other drugs, such as selective ...
-
6 ADVERSE REACTIONSThe following clinically significant reactions are described in greater detail, in other sections. Serotonin Syndrome [see Warnings and Precautions (5.1)] Adverse Cardiovascular Effects [see ...
-
7 DRUG INTERACTIONSBased on its structural similarity to tricyclic antidepressants, cyclobenzaprine hydrochloride extended-release capsules may have life-threatening interactions with MAO inhibitors [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from case reports with cyclobenzaprine hydrochloride extended-release capsules use in pregnancy have not identified a drug-associated risk of major ...
-
9 DRUG ABUSE AND DEPENDENCE9.3 Dependence - Pharmacologic similarities among the tricyclic drugs require that certain withdrawal symptoms be considered when cyclobenzaprine hydrochloride extended-release capsules are ...
-
10 OVERDOSAGEClinical Presentation - Although rare, deaths may occur from overdosage with cyclobenzaprine hydrochloride extended-release capsules. Multiple drug ingestion (including alcohol) is common in ...
-
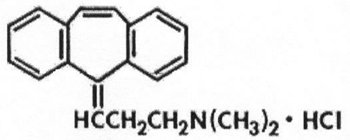
11 DESCRIPTIONCyclobenzaprine hydrochloride is a skeletal muscle relaxant which relieves muscle spasm of local origin without interfering with muscle function. The active ingredient in cyclobenzaprine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Cyclobenzaprine relieves skeletal muscle spasm of local origin without interfering with muscle function. Cyclobenzaprine has not been shown to be effective in muscle ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies were conducted in CD-1 mice and Sprague-Dawley rats with oral cyclobenzaprine to evaluate its ...
-
14 CLINICAL STUDIESEfficacy was assessed in two double-blind, parallel-group, active-controlled, placebo-controlled studies of identical design of cyclobenzaprine hydrochloride extended-release 15 mg and 30 mg ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCyclobenzaprine hydrochloride extended-release 15 mg capsules are orange/orange and are embossed in blue ink with “15 mg” on the body, and Cephalon “C” logo, “Cephalon”, and a dashed band on the ...
-
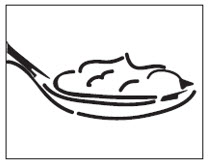
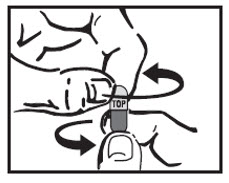
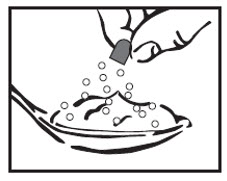
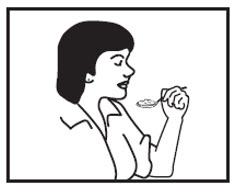
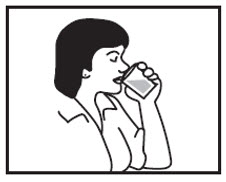
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information). Instruct patients to swallow cyclobenzaprine hydrochloride extended-release capsules intact or to sprinkle capsule contents on a ...
-
Patient InformationPATIENT INFORMATION - Cyclobenzaprine Hydrochloride Extended-Release Capsules - What are cyclobenzaprine hydrochloride extended-release capsules? Cyclobenzaprine hydrochloride ...
-
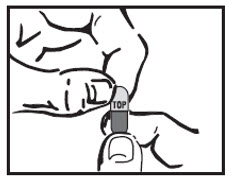
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - Cyclobenzaprine Hydrochloride Extended-Release Capsules - Read this Instructions for Use before you prepare your first dose of cyclobenzaprine hydrochloride extended-release ...
-
PRINCIPAL DISPLAY PANELCyclobenzaprine Hydrochloride Extended-Release 15mg Capsules
-
INGREDIENTS AND APPEARANCEProduct Information