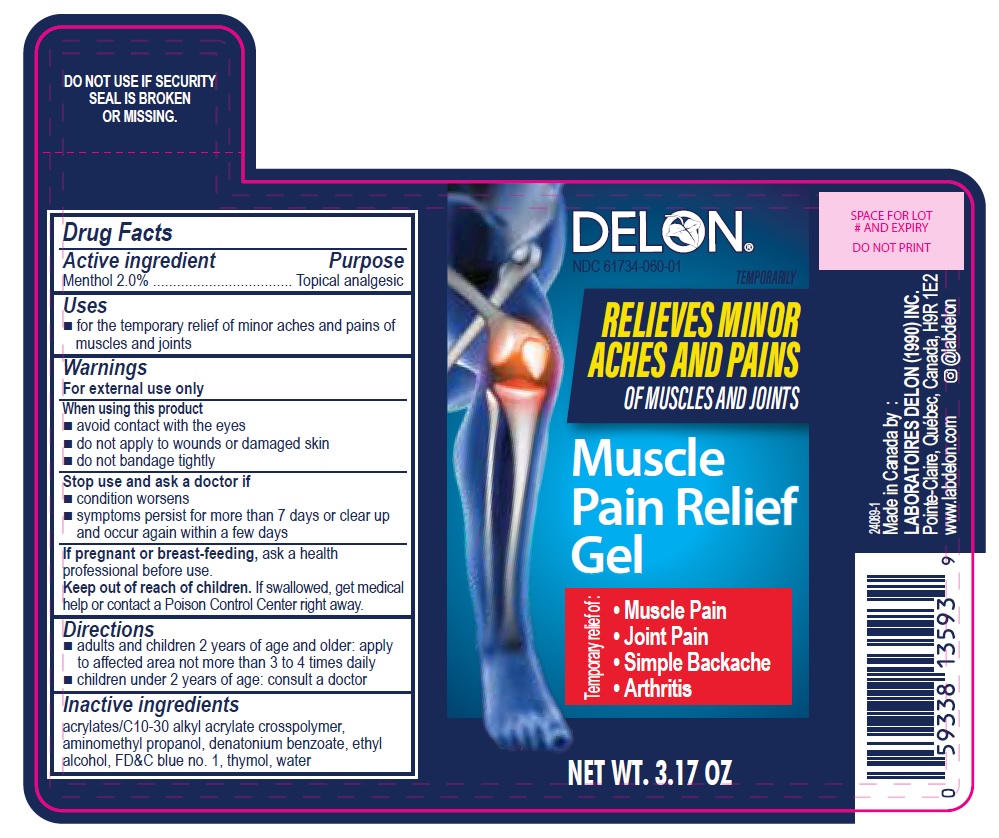
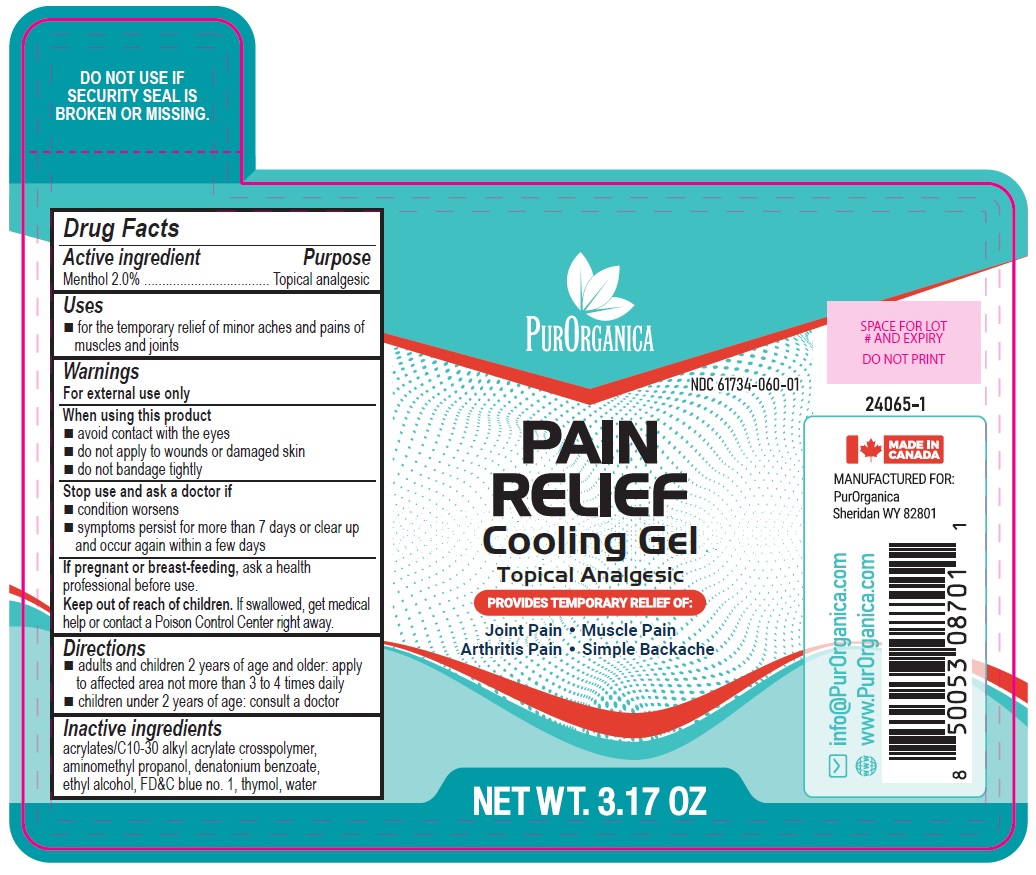
Label: MUSCLE PAIN RELIEF GEL- menthol gel
- NDC Code(s): 61734-060-01
- Packager: Delon Laboratories (1990) Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- DELON Muscle Pain Relief Gel 3.17 OZ
- PURORGANICA PAIN RELIEF Cooling Gel Topical Analgesic 3.17 OZ
-
INGREDIENTS AND APPEARANCE
MUSCLE PAIN RELIEF GEL
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61734-060 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2 g in 100 g Inactive Ingredients Ingredient Name Strength DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) ALCOHOL (UNII: 3K9958V90M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) THYMOL (UNII: 3J50XA376E) WATER (UNII: 059QF0KO0R) Product Characteristics Color blue Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61734-060-01 90 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 09/09/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/09/2024 Labeler - Delon Laboratories (1990) Ltd. (248364184) Establishment Name Address ID/FEI Business Operations Laboratoires Delon 208896216 manufacture(61734-060) , pack(61734-060) , label(61734-060)