Label: STERILE WATER- water injection, solution
- NDC Code(s): 0338-0013-06, 0338-0013-08, 0338-0013-29
- Packager: Baxter Healthcare Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 31, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONSterile Water for Injection, USP is sterile, nonpyrogenic, distilled water in a Pharmacy Bulk Package. A Pharmacy Bulk Package is a container of a sterile preparation for parenteral use that ...
-
CLINICAL PHARMACOLOGYSterile Water for Injection, USP is used for fluid replacement only after suitable admixing to approximate isotonicity.
-
INDICATIONS AND USAGESterile Water for Injection, USP is indicated in the aseptic preparation of parenteral admixtures.
-
CONTRAINDICATIONSSterile Water for Injection, USP is a hemolytic agent due to its hypotonicity. Therefore, it is contraindicated for intravenous administration without admixing.
-
WARNINGSThis solution is for compounding only, not for direct infusion. Hemolysis may occur following infusion of Sterile Water for Injection, USP. Hemoglobin induced renal failure has been reported ...
-
PRECAUTIONSDo not use unless solution is clear and seal is intact. Drug product contains no more than 25 µg/L of aluminum. Pediatric Use: Safety and effectiveness have been established in pediatric ...
-
ADVERSE REACTIONSThe administration of a suitable admixture of prescribed drugs may be associated with adverse reactions because of the solution or the technique of administration including febrile response ...
-
DOSAGE AND ADMINISTRATIONFollowing suitable admixture of prescribed drugs, the dosage is usually dependent upon the age, weight and clinical condition of the patient as well as laboratory determinations. See directions ...
-
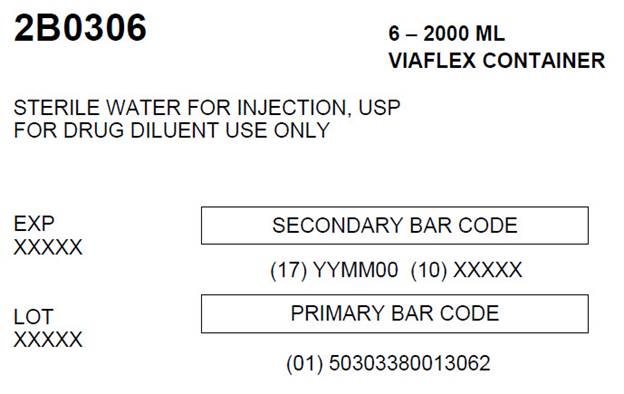
HOW SUPPLIEDSterile Water for Injection, USP is supplied in a VIAFLEX plastic Pharmacy Bulk Package container as follows: 2000 mL - 2B0306 - NDC 0338-0013-06 - 3000 mL - 2B0307 - NDC ...
-
PACKAGE LABEL - PRINCIPLE DISPLAY PANELContainer Label - Container Label - LOT EXP - 2B0306 2000 mL - NDC 0338-0013-06 DIN 02014882 - Sterile Water - For Injection USP - Pharmacy Bulk Package - Not For Direct Infusion - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information