Label: NATACYN- natamycin suspension/ drops
- NDC Code(s): 82667-012-05
- Packager: Harrow Eye, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
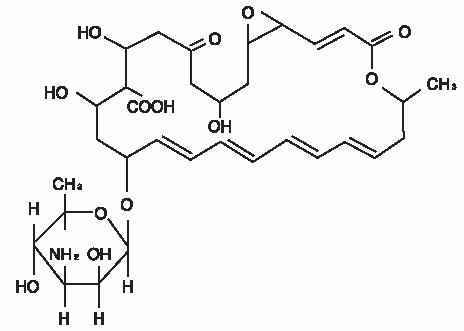
DESCRIPTION:NATACYN™ (natamycin ophthalmic suspension) 5% is a sterile, antifungal drug for topical ophthalmic administration. Each mL of NATACYN™ (natamycin ophthalmic suspension) contains: Active ...
-
CLINICAL PHARMACOLOGY:Natamycin is a tetraene polyene antibiotic derived from - Streptomyces natalensis. It possesses - in vitro activity against a variety of yeast and filamentous fungi, including - Candida ...
-
INDICATIONS AND USAGE:NATACYN™ (natamycin ophthalmic suspension) 5% is indicated for the treatment of fungal blepharitis, conjunctivitis, and keratitis caused by susceptible organisms including - Fusarium solani ...
-
CONTRAINDICATIONS:NATACYN™ (natamycin ophthalmic suspension) 5% is contraindicated in individuals with a history of hypersensitivity to any of its components.
-
PRECAUTIONS:
General.
FOR TOPICAL OPHTHALMIC USE ONLY — NOT FOR INJECTION. Failure of improvement of keratitis following 7-10 days of administration of the drug suggests that the infection may be caused by a ...
-
Information for Patients:Do not touch dropper tip to any surface, as this may contaminate the suspension. Patients should be advised not to wear contact lenses if they have signs and symptoms of fungal blepharitis ...
-
Carcinogenesis, Mutagenesis, Impairment of Fertility:There have been no long term studies done using natamycin in animals to evaluate carcinogenesis, mutagenesis, or impairment of fertility.
-
Pregnancy:Animal reproduction studies have not been conducted with natamycin. It is also not known whether natamycin can cause fetal harm when administered to a pregnant woman or can affect reproduction ...
-
Nursing Mothers:It is not known whether these drugs are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when natamycin is administered to a nursing woman.
-
Pediatric Use:Safety and effectiveness in pediatric patients have not been established.
-
Geriatric Use:No overall differences in safety or effectiveness have been observed between elderly and younger patients.
-
ADVERSE REACTIONS:The following events have been identified during post-marketing use of NATACYN - ™ (natamycin ophthalmic suspension) 5% in clinical practice. Because they are reported voluntarily from a ...
-
DOSAGE AND ADMINISTRATION:SHAKE WELL BEFORE USING. The preferred initial dosage in fungal keratitis is one drop of NATACYN™ (natamycin ophthalmic suspension) 5% instilled in the conjunctival sac at hourly or two-hourly ...
-
HOW SUPPLIED:NATACYN™ (natamycin ophthalmic suspension) 5% is a 15 mL fill packaged in a 15 mL amber glass bottle with a black closure. A flint glass dropper with a red plastic closure and a black rubber bulb ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Harrow Eye, LLC™ Nashville, TN USA - Revised: 07/2024
-
PRINCIPAL DISPLAY PANELNDC 82667-012-05 - Harrow - ® Natacyn™ (natamycin ophthalmic suspension) 5% Anti-Fungal Ophthalmic Suspension - Rx Only - 15 mL - Sterile
-
INGREDIENTS AND APPEARANCEProduct Information