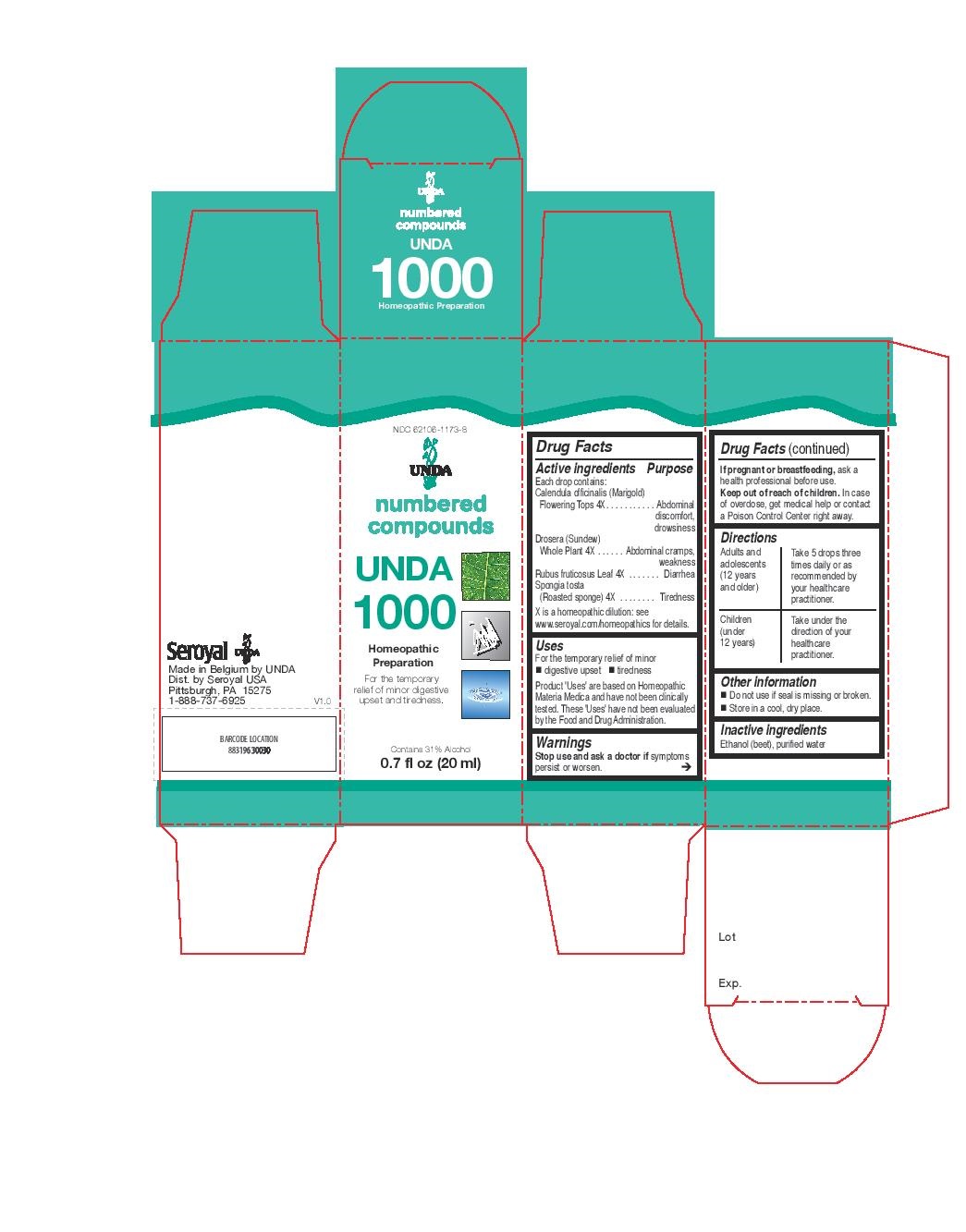
Label: UNDA 1000- rubus fruticosus, spongia tosta, calendula officinalis, drosera liquid
- NDC Code(s): 62106-1173-8
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 9, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- STOP USE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 1000
rubus fruticosus, spongia tosta, calendula officinalis, drosera liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1173 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RUBUS FRUTICOSUS LEAF (UNII: YQ2S06L8S9) (RUBUS FRUTICOSUS LEAF - UNII:YQ2S06L8S9) RUBUS FRUTICOSUS LEAF 4 [hp_X] in 20 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 4 [hp_X] in 20 mL DROSERA ROTUNDIFOLIA FLOWERING TOP (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA FLOWERING TOP - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA FLOWERING TOP 4 [hp_X] in 20 mL SPONGIA OFFICINALIS SKELETON, ROASTED (UNII: 1PIP394IID) (SPONGIA OFFICINALIS SKELETON, ROASTED - UNII:1PIP394IID) SPONGIA OFFICINALIS SKELETON, ROASTED 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1173-8 1 in 1 CARTON 09/23/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/23/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1173)