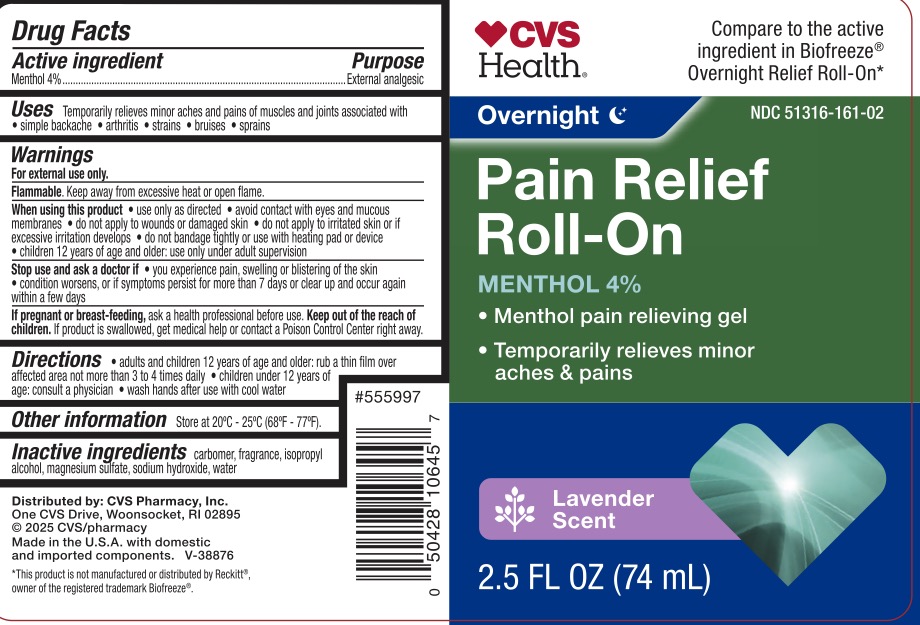
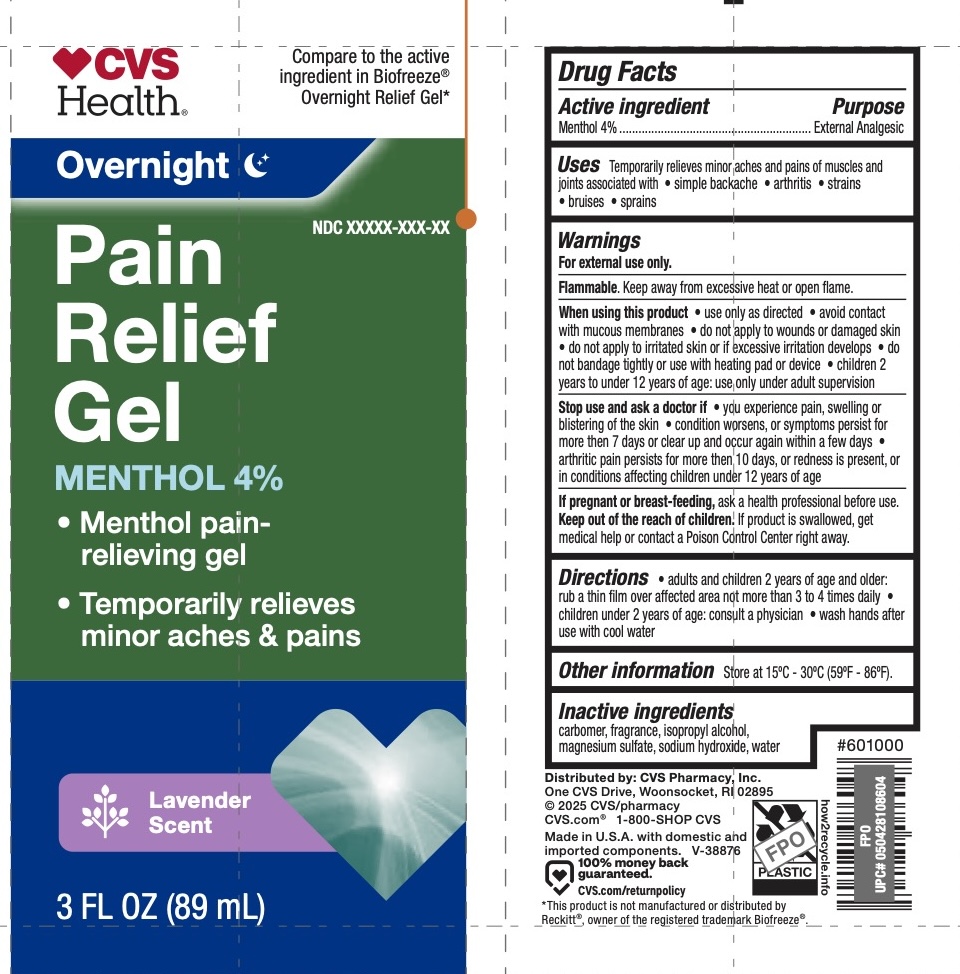
Label: CVS PAIN RELIEF- menthol 4% gel
- NDC Code(s): 51316-161-02, 51316-161-03
- Packager: CVS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For external use only.
Flammable. Keep away from excessive heat or open flame.
When using this product use only as directed. Avoid contact with eyes and mucous membranes, do not apply to wounds or damaged skin, do no apply to irritated skin or if excessive irritation develops, do not bandage tightly or use with heating pad or device. Children 12 years of age and older: use only under adult supervision.
Stop use and ask a doctor if you experience pain, swelling, or blistering of the skin, condition worsens, symptoms last more than 7 days or clear up and occur again within a few days.
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS PAIN RELIEF
menthol 4% gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51316-161 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, (+)- (UNII: C6B1OE8P3W) (MENTHOL, (+)- - UNII:C6B1OE8P3W) MENTHOL, (+)- 4 g in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER 940 (UNII: 4Q93RCW27E) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51316-161-02 74 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/15/2024 2 NDC:51316-161-03 89 mL in 1 TUBE; Type 0: Not a Combination Product 11/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/15/2024 Labeler - CVS (062312574) Registrant - Derma Care Research Labs, LLC (116817470) Establishment Name Address ID/FEI Business Operations Derma Care Research Labs, LLC 116817470 manufacture(51316-161)