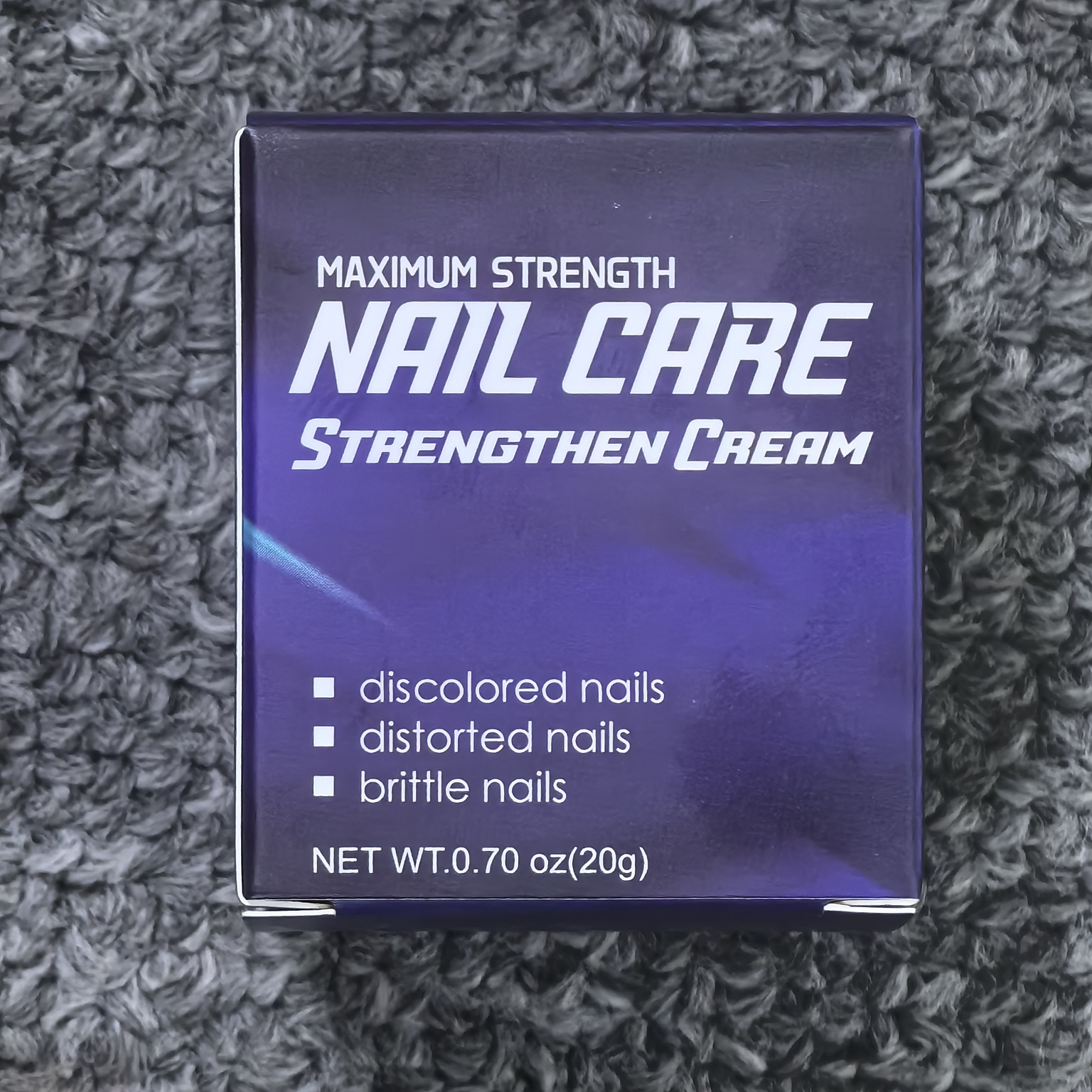
Label: NAIL STRENGTHEN CREAM cream
- NDC Code(s): 84117-022-01
- Packager: Shenzhen Hengkaifeng Commerce and Trade Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- PURPOSE
- Uses
-
Warnings
For external use only, some people may be sensitive to essential oils.
to determine if the product may cause any irritation. Discontinue use if any irritation occurs.
If condition worsens, discontinue use and consult a physician. Not suitable for use by children under 2 years of age without a physician's guidance. - Stop use and ask a doctor if
- Do not use
- When using this product
- Keep out of reach of children.
- inactive ingredients
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NAIL STRENGTHEN CREAM
nail strengthen cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84117-022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 100 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84117-022-01 20 g in 1 CANISTER; Type 0: Not a Combination Product 08/12/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 08/12/2024 Labeler - Shenzhen Hengkaifeng Commerce and Trade Co., Ltd (444722774) Establishment Name Address ID/FEI Business Operations Shenzhen Hengkaifeng Commerce and Trade Co., Ltd 444722774 label(84117-022) , manufacture(84117-022)