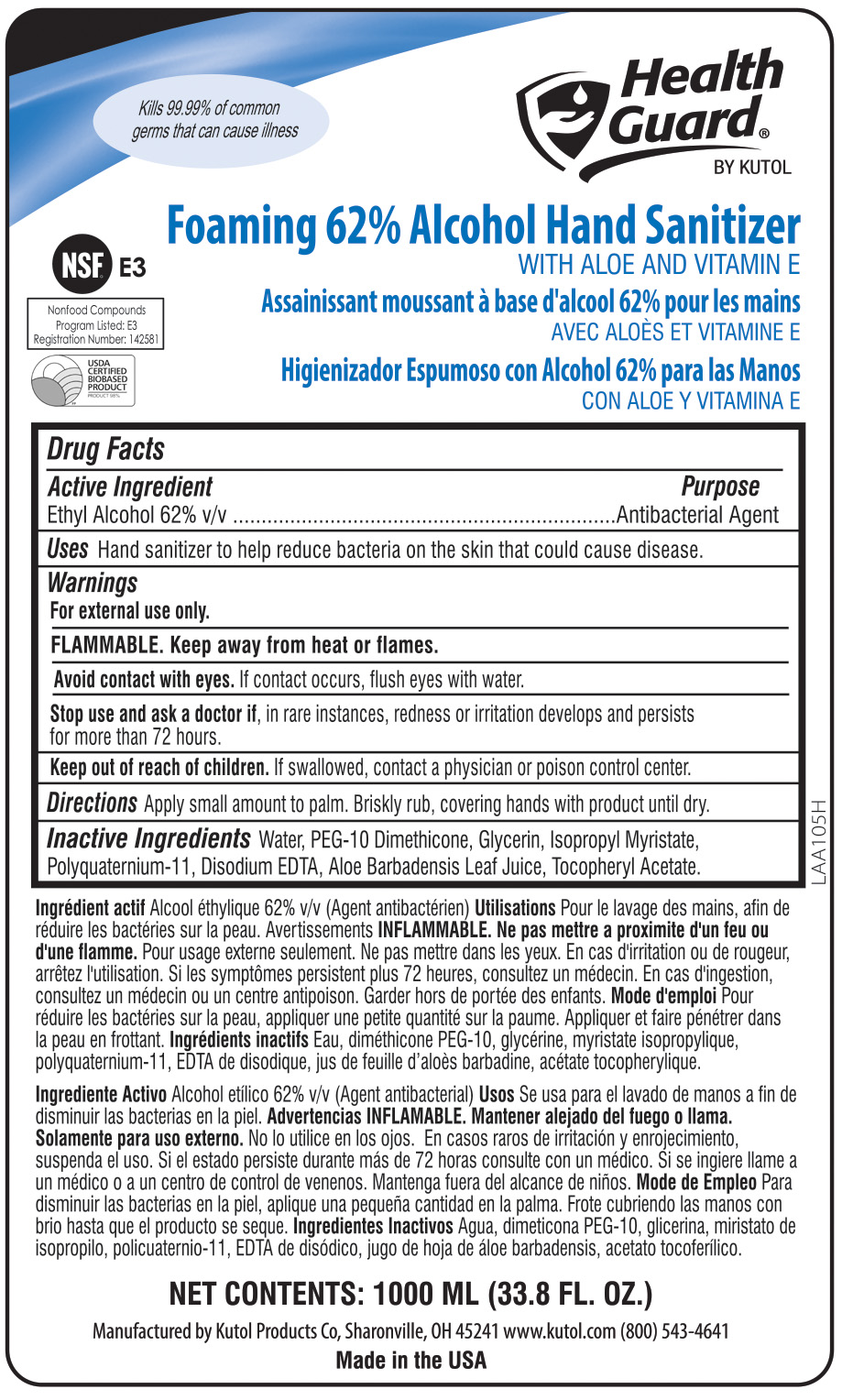
Label: EZFOAM FOAMING ALCOHOL HAND SANITIZER- foaming hand sanitizer solution
-
NDC Code(s):
50865-688-03,
50865-688-09,
50865-688-17,
50865-688-24, view more50865-688-41, 50865-688-50, 50865-688-78, 50865-688-91
- Packager: Kutol Products Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
For external use only.
FLAMMABLE. Keep away from heat or flames.
Avoid contact with eyes. If eye contact occurs, flush with water.
Stop use if, in rare instances, redness or irritation develop. If condition persists for more than 72 hours, consult a physician.
Keep out of reach of children. If swallowed, contact a physician or poison control center.
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Hand sanitizer to help reduce bacteria on the skin that could cause disease. Recommended for repeated use.
Avoid contact with eyes. If eye contact occurs, flush with water.
Stop use if, in rare instances, redness or irritation develop. If condition persists for more than 72 hours, consult a physician.
Keep out of reach of children. If swallowed, contact a physician or poison control center.
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EZFOAM FOAMING ALCOHOL HAND SANITIZER
foaming hand sanitizer solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50865-688 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) AMINOMETHYLPROPANOL (PERFLUORO-C6-C12 ETHYL)PHOSPHATE (UNII: QCD5R22RNT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50865-688-03 213125 mL in 1 DRUM; Type 0: Not a Combination Product 06/27/2013 01/21/2016 2 NDC:50865-688-09 3785 mL in 1 JUG; Type 0: Not a Combination Product 06/27/2013 3 NDC:50865-688-17 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/27/2013 4 NDC:50865-688-24 1000 mL in 1 BAG; Type 0: Not a Combination Product 06/27/2013 5 NDC:50865-688-41 1000 mL in 1 BAG; Type 0: Not a Combination Product 06/27/2013 6 NDC:50865-688-78 950 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/27/2013 7 NDC:50865-688-91 532 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/27/2013 01/21/2016 8 NDC:50865-688-50 1200 mL in 1 BAG; Type 0: Not a Combination Product 06/27/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/27/2013 Labeler - Kutol Products Company (004236139) Registrant - Kutol Products Company. (004236139) Establishment Name Address ID/FEI Business Operations Kutol Products Company 004236139 manufacture(50865-688)